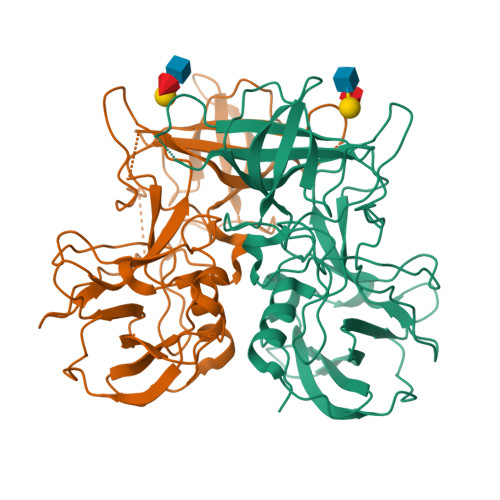
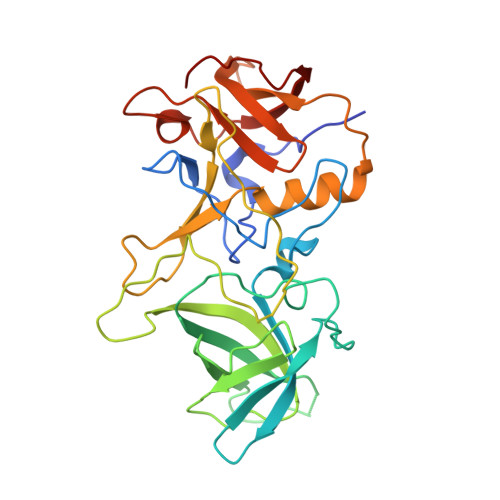
Structural analysis of determinants of histo-blood group antigen binding specificity in genogroup I noroviruses.
Shanker, S., Czako, R., Sankaran, B., Atmar, R.L., Estes, M.K., Prasad, B.V.(2014) J Virol 88: 6168-6180
- PubMed: 24648450
- DOI: https://doi.org/10.1128/JVI.00201-14
- Primary Citation of Related Structures:
4P12, 4P1V, 4P25, 4P26, 4P2N, 4P3I - PubMed Abstract:
Human noroviruses (NoVs) cause acute epidemic gastroenteritis. Susceptibility to the majority of NoV infections is determined by genetically controlled secretor-dependent expression of histo-blood group antigens (HBGAs), which are also critical for NoV attachment to host cells. Human NoVs are classified into two major genogroups (genogroup I [GI] and GII), with each genogroup further divided into several genotypes. GII NoVs are more prevalent and exhibit periodic emergence of new variants, suggested to be driven by altered HBGA binding specificities and antigenic drift. Recent epidemiological studies show increased activity among GI NoVs, with some members showing the ability to bind nonsecretor HBGAs. NoVs bind HBGAs through the protruding (P) domain of the major capsid protein VP1. GI NoVs, similar to GII, exhibit significant sequence variations in the P domain; it is unclear how these variations affect HBGA binding specificities. To understand the determinants of possible strain-specific HBGA binding among GI NoVs, we determined the structure of the P domain of a GI.7 clinical isolate and compared it to the previously determined P domain structures of GI.1 and GI.2 strains. Our crystallographic studies revealed significant structural differences, particularly in the loop regions of the GI.7 P domain, altering its surface topography and electrostatic landscape and potentially indicating antigenic variation. The GI.7 strain bound to H- and A-type, Lewis secretor, and Lewis nonsecretor families of HBGAs, allowing us to further elucidate the structural determinants of nonsecretor HBGA binding among GI NoVs and to infer several contrasting and generalizable features of HBGA binding in the GI NoVs. Human noroviruses (NoVs) cause acute epidemic gastroenteritis. Recent epidemiological studies have shown increased prevalence of genogroup I (GI) NoVs. Although secretor-positive status is strongly correlated with NoV infection, cases of NoV infection associated with secretor-negative individuals are reported. Biochemical studies have shown that GI NoVs exhibit genotype-dependent binding to nonsecretor histo-blood group antigens (HBGAs). From our crystallographic studies of a GI.7 NoV, in comparison with previous studies on GI.1 and GI.2 NoVs, we show that genotypic differences translate to extensive structural changes in the loop regions that significantly alter the surface topography and electrostatic landscape of the P domain; these features may be indicative of antigenic variations contributing to serotypic differentiation in GI NoVs and also differential modulation of the HBGA binding characteristics. A significant finding is that the threshold length and the structure of one of the loops are critical determinants in the binding of GI NoVs to nonsecretor HBGAs.
Organizational Affiliation:
Verna Marrs McLean Department of Biochemistry and Molecular Biology, Baylor College of Medicine, Houston, Texas, USA.