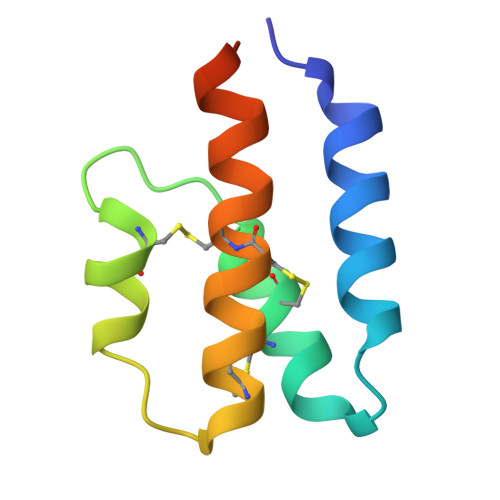
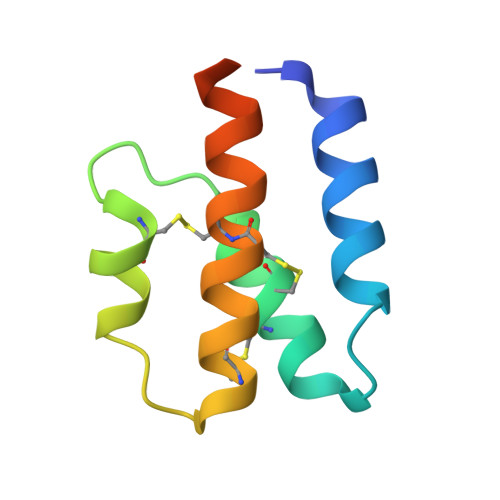
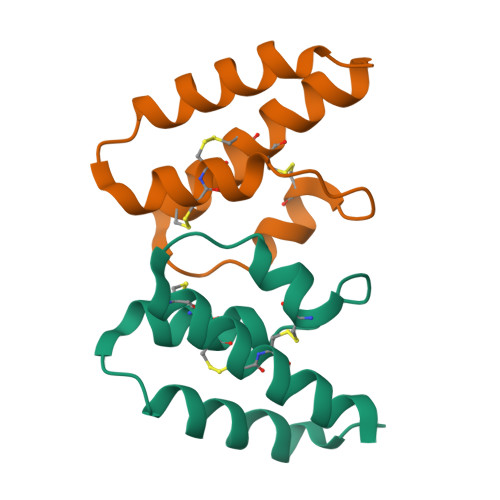
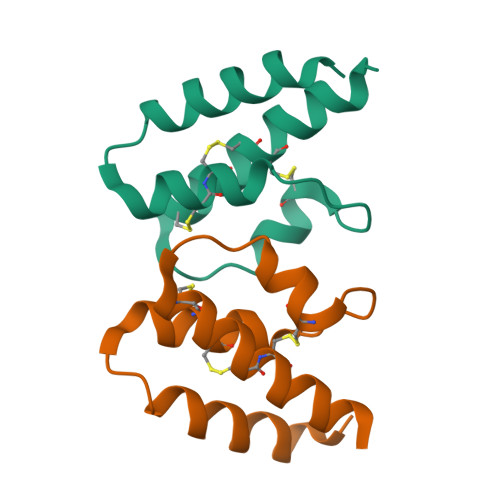
Structural and functional characterization of human and murine C5a anaphylatoxins.
Schatz-Jakobsen, J.A., Yatime, L., Larsen, C., Petersen, S.V., Klos, A., Andersen, G.R.(2014) Acta Crystallogr D Biol Crystallogr 70: 1704-1717
- PubMed: 24914981
- DOI: https://doi.org/10.1107/S139900471400844X
- Primary Citation of Related Structures:
4P39, 4P3A, 4P3B - PubMed Abstract:
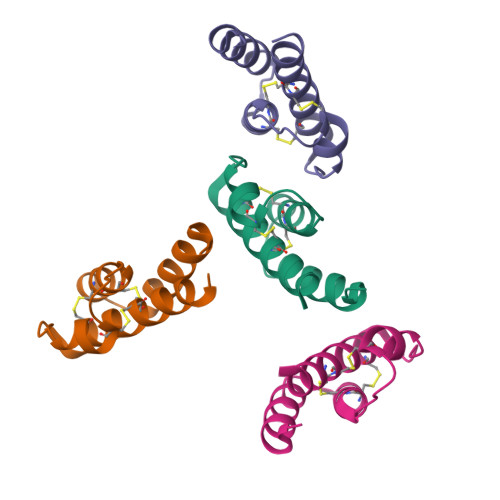
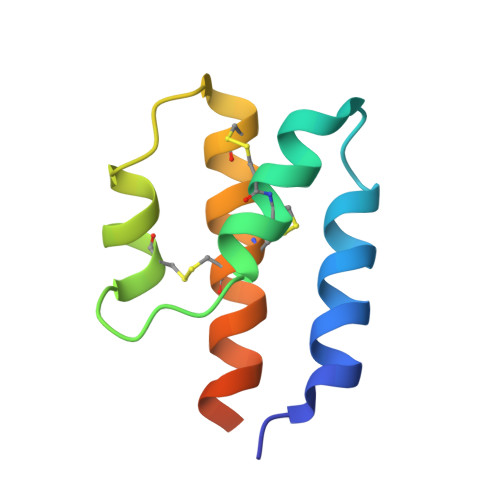
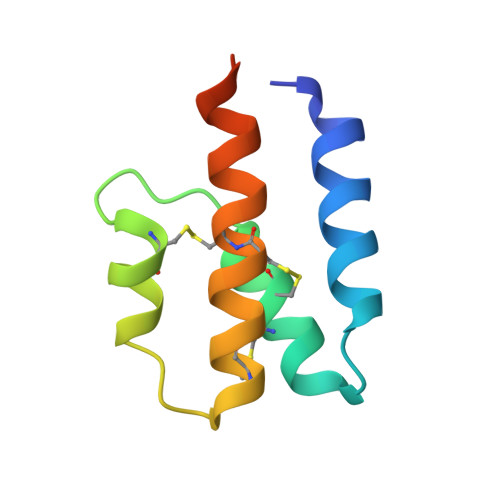
Complement is an ancient part of the innate immune system that plays a pivotal role in protection against invading pathogens and helps to clear apoptotic and necrotic cells. Upon complement activation, a cascade of proteolytic events generates the complement effectors, including the anaphylatoxins C3a and C5a. Signalling through their cognate G-protein coupled receptors, C3aR and C5aR, leads to a wide range of biological events promoting inflammation at the site of complement activation. The function of anaphylatoxins is regulated by circulating carboxypeptidases that remove their C-terminal arginine residue, yielding C3a-desArg and C5a-desArg. Whereas human C3a and C3a-desArg adopt a canonical four-helix bundle fold, the conformation of human C5a-desArg has recently been described as a three-helix bundle. Here, the crystal structures of an antagonist version of human C5a, A8(Δ71-73), and of murine C5a and C5a-desArg are reported. Whereas A8(Δ71-73) adopts a three-helix bundle conformation similar to human C5a-desArg, the two murine proteins form a four-helix bundle. A cell-based functional assay reveals that murine C5a-desArg, in contrast to its human counterpart, exerts the same level of activition as murine C5a on its cognate receptor. The role of the different C5a conformations is discussed in relation to the differential activation of C5a receptors across species.
Organizational Affiliation:
Department of Molecular Biology and Genetics, Aarhus University, Gustav Wieds Vej 10C, DK-8000 Aarhus, Denmark.