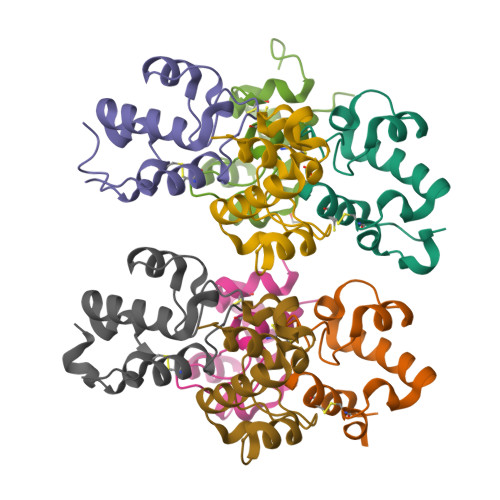
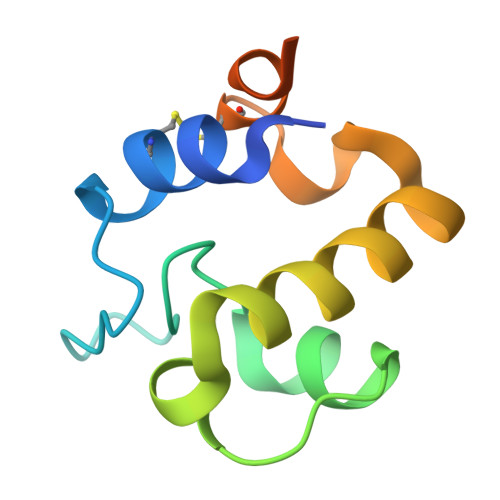
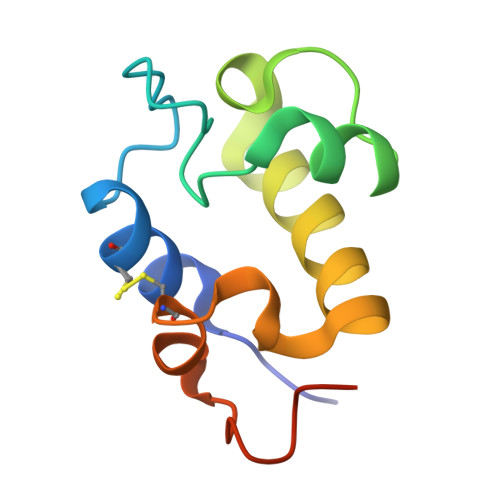
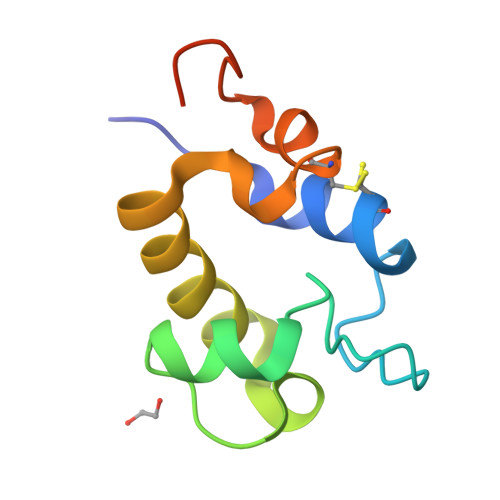
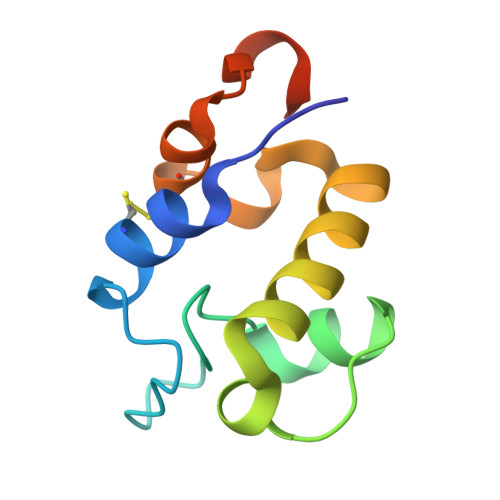
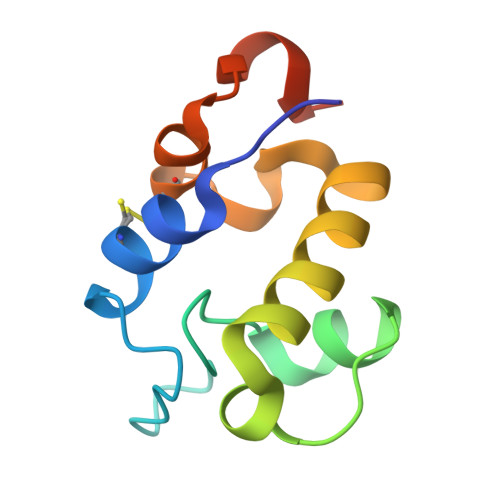
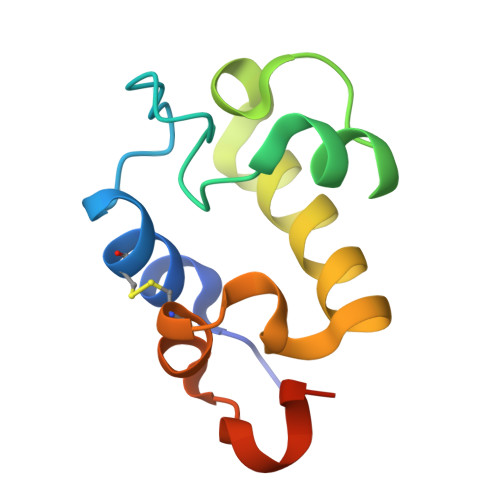
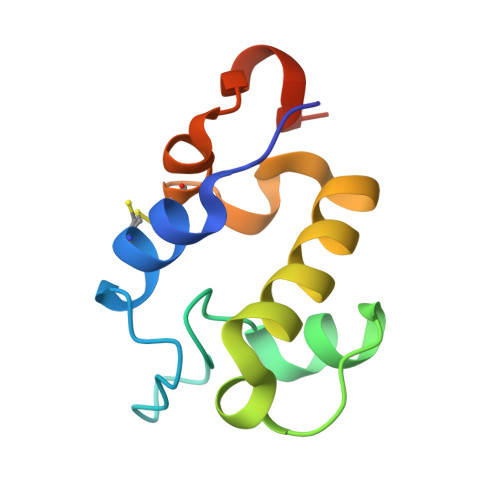
The RpfC (Rv1884) atomic structure shows high structural conservation within the resuscitation-promoting factor catalytic domain.
Chauviac, F.X., Robertson, G., Quay, D.H., Bagneris, C., Dumas, C., Henderson, B., Ward, J., Keep, N.H., Cohen-Gonsaud, M.(2014) Acta Crystallogr F Struct Biol Commun 70: 1022-1026
- PubMed: 25084374
- DOI: https://doi.org/10.1107/S2053230X1401317X
- Primary Citation of Related Structures:
4OW1 - PubMed Abstract:
The first structure of the catalytic domain of RpfC (Rv1884), one of the resuscitation-promoting factors (RPFs) from Mycobacterium tuberculosis, is reported. The structure was solved using molecular replacement once the space group had been correctly identified as twinned P21 rather than the apparent C2221 by searching for anomalous scattering sites in P1. The structure displays a very high degree of structural conservation with the previously published structures of the catalytic domains of RpfB (Rv1009) and RpfE (Rv2450). This structural conservation highlights the importance of the versatile domain composition of the RPF family.
Organizational Affiliation:
Crystallography, Institute for Structural and Molecular Biology, Department of Biological Sciences, Birkbeck, University of London, Malet Street, London WC1E 7HX, England.