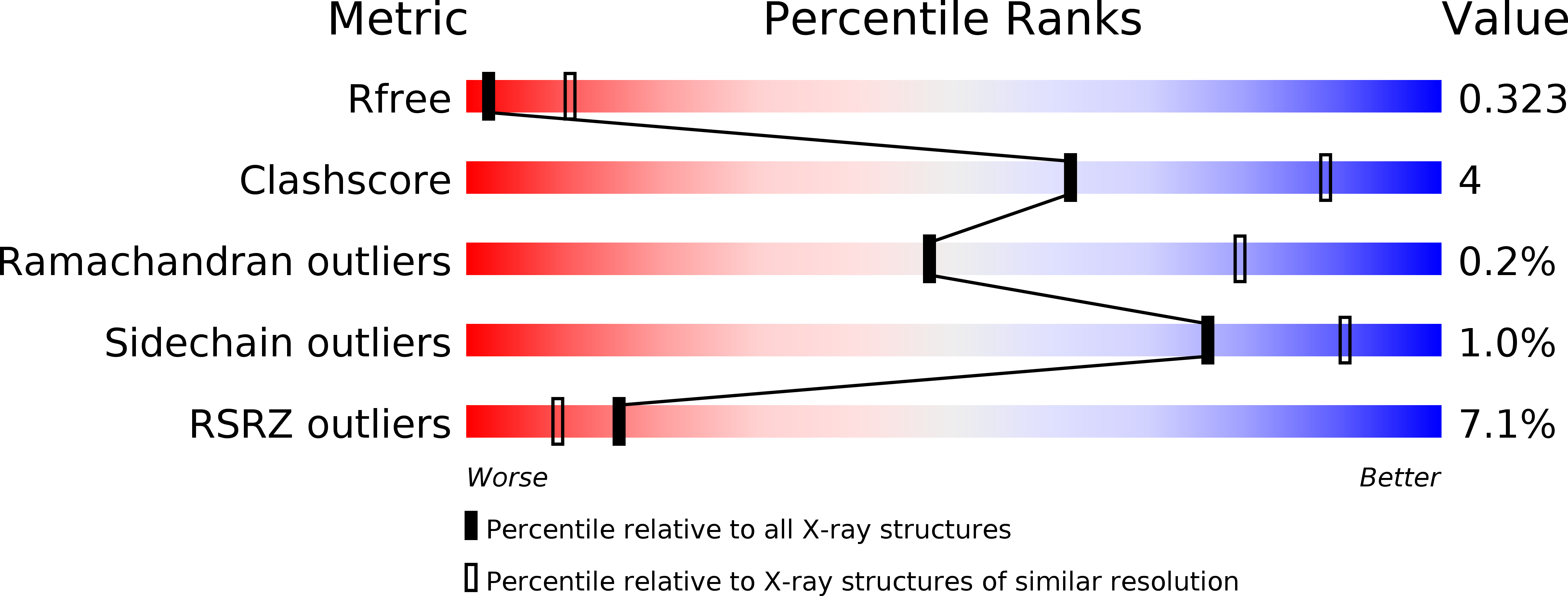
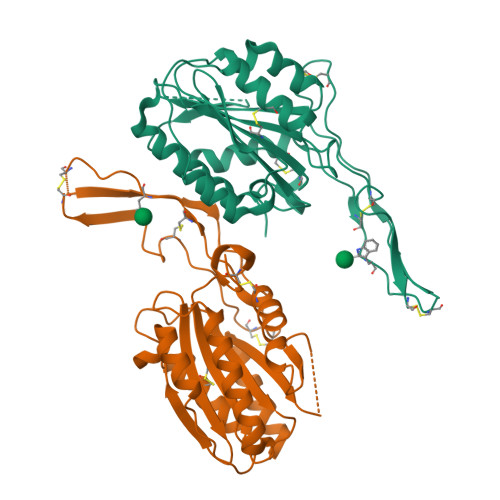
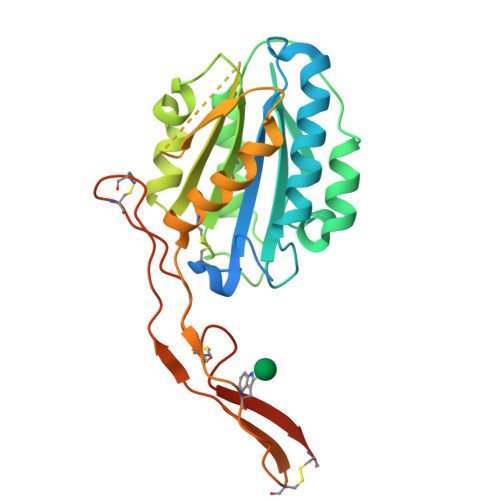
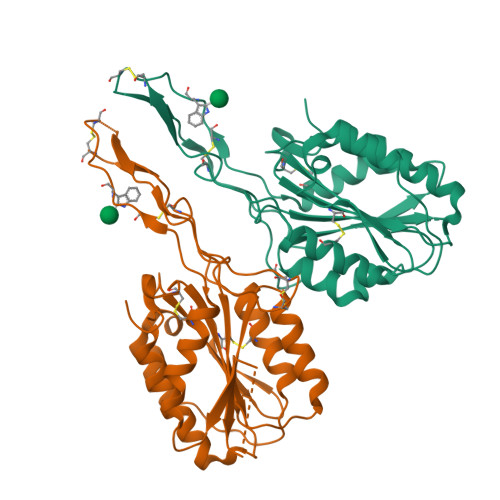
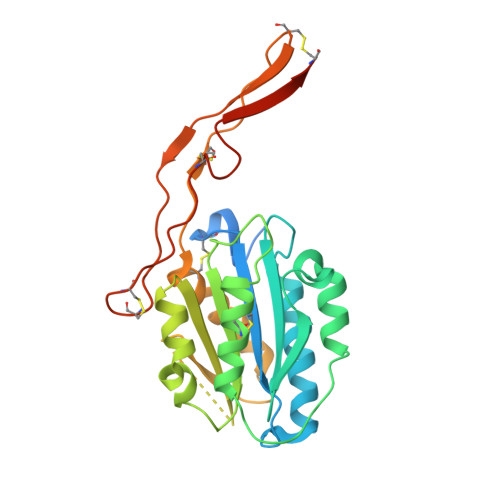
Structures of the Toxoplasma gliding motility adhesin.
Song, G., Springer, T.A.(2014) Proc Natl Acad Sci U S A 111: 4862-4867
- PubMed: 24639528
- DOI: https://doi.org/10.1073/pnas.1403059111
- Primary Citation of Related Structures:
4OKR, 4OKU - PubMed Abstract:
Micronemal protein 2 (MIC2) is the key adhesin that supports gliding motility and host cell invasion by Toxoplasma gondii. With a von Willebrand factor A (VWA) domain and six thrombospondin repeat domains (TSR1-6) in its ectodomain, MIC2 connects to the parasite actomyosin system through its cytoplasmic tail. MIC2-associated protein (M2AP) binds noncovalently to the MIC2 ectodomain. MIC2 and M2AP are stored in micronemes as proforms. We find that the MIC2-M2AP ectodomain complex is a highly elongated 1:1 monomer with M2AP bound to the TSR6 domain. Crystal structures of N-terminal fragments containing the VWA and TSR1 domains for proMIC2 and MIC2 reveal a closed conformation of the VWA domain and how it associates with the TSR1 domain. A long, proline-rich, disulfide-bonded pigtail loop in TSR1 overlaps the VWA domain. Mannose α-C-linked to Trp-276 in TSR1 has an unusual (1)C4 chair conformation. The MIC2 VWA domain includes a mobile α5-helix and a 22-residue disordered region containing two disulfide bonds in place of an α6-helix. A hydrophobic residue in the prodomain binds to a pocket adjacent to the α7-helix that pistons in opening of the VWA domain to a putative high-affinity state.
Organizational Affiliation:
Program in Cellular and Molecular Medicine and Division of Hematology, Department of Medicine, Boston Children's Hospital, Boston, MA 02115.