Identification of Novel HSP90 alpha / beta Isoform Selective Inhibitors Using Structure-Based Drug Design. Demonstration of Potential Utility in Treating CNS Disorders such as Huntington's Disease.
Ernst, J.T., Neubert, T., Liu, M., Sperry, S., Zuccola, H., Turnbull, A., Fleck, B., Kargo, W., Woody, L., Chiang, P., Tran, D., Chen, W., Snyder, P., Alcacio, T., Nezami, A., Reynolds, J., Alvi, K., Goulet, L., Stamos, D.(2014) J Med Chem 57: 3382-3400
- PubMed: 24673104
- DOI: https://doi.org/10.1021/jm500042s
- Primary Citation of Related Structures:
4O04, 4O05, 4O07, 4O09, 4O0B - PubMed Abstract:
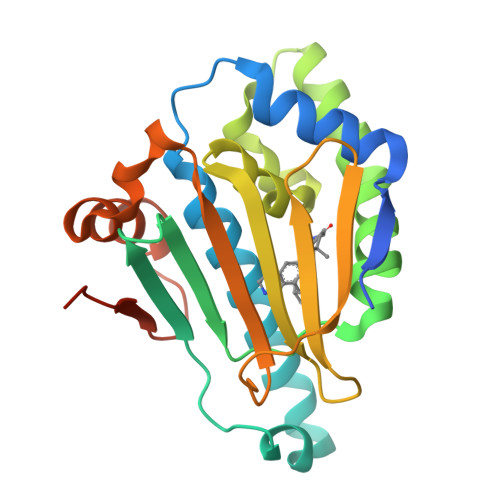
A structure-based drug design strategy was used to optimize a novel benzolactam series of HSP90α/β inhibitors to achieve >1000-fold selectivity versus the HSP90 endoplasmic reticulum and mitochondrial isoforms (GRP94 and TRAP1, respectively). Selective HSP90α/β inhibitors were found to be equipotent to pan-HSP90 inhibitors in promoting the clearance of mutant huntingtin protein (mHtt) in vitro, however with less cellular toxicity. Improved tolerability profiles may enable the use of HSP90α/β selective inhibitors in treating chronic neurodegenerative indications such as Huntington's disease (HD). A potent, selective, orally available HSP90α/β inhibitor was identified (compound 31) that crosses the blood-brain barrier. Compound 31 demonstrated proof of concept by successfully reducing brain Htt levels following oral dosing in rats.
Organizational Affiliation:
Vertex Pharmaceuticals , 11010 Torreyana Road, San Diego, California 92121, United States.