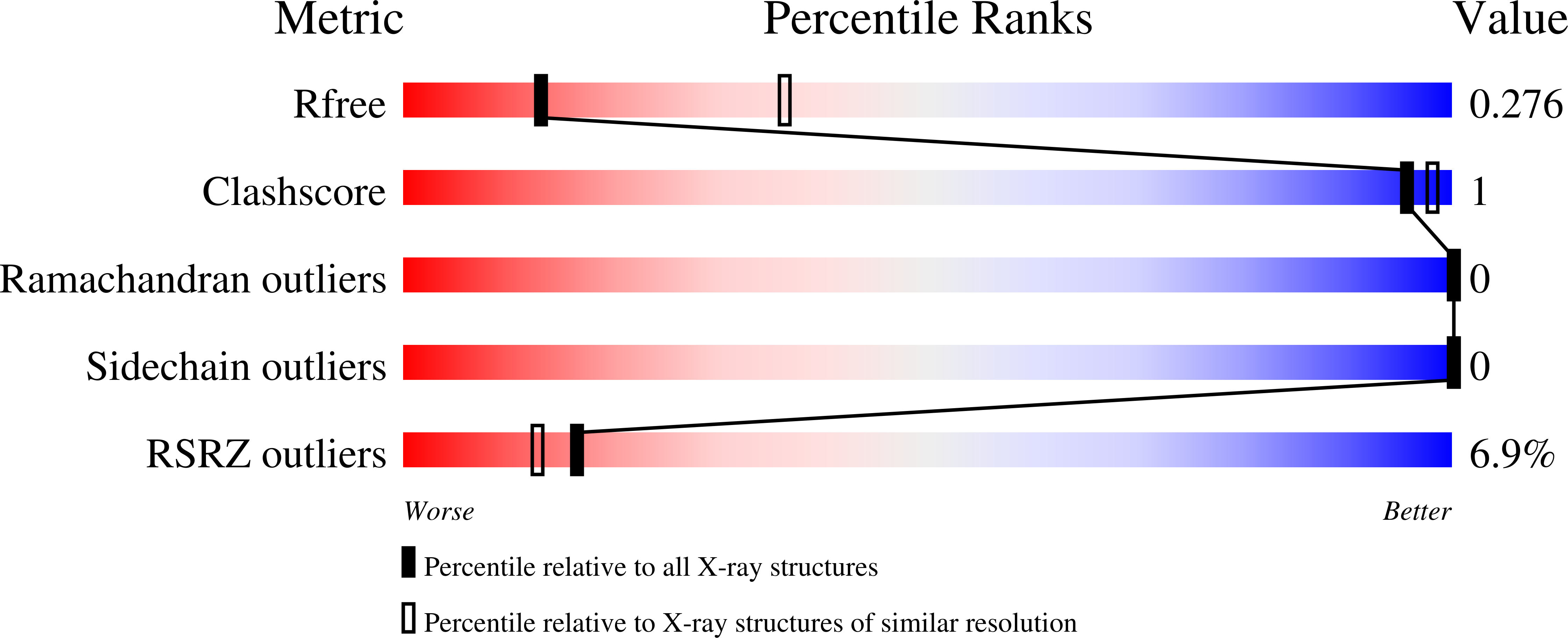
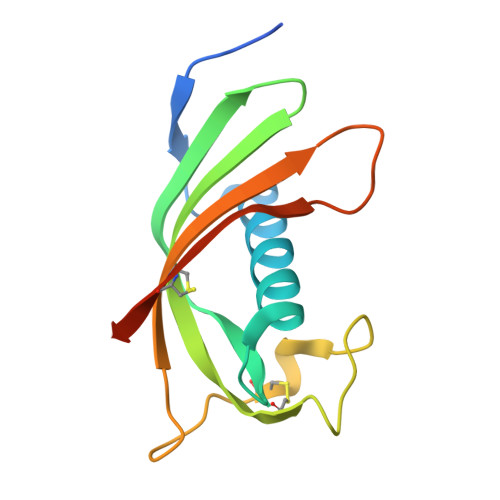
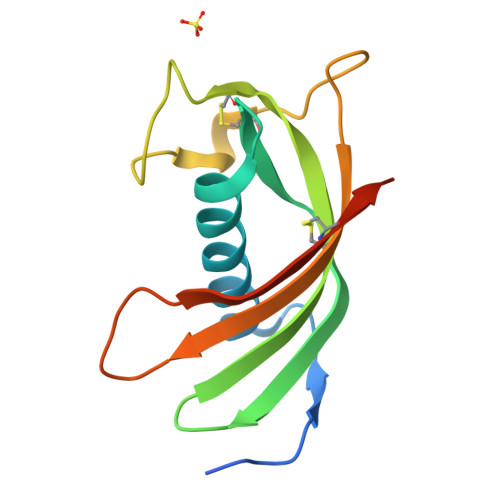
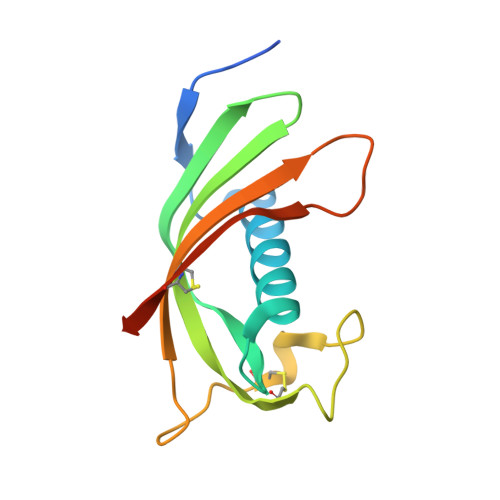
Structure and mechanism of an aspartimide-dependent Peptide ligase in human legumain.
Dall, E., Fegg, J.C., Briza, P., Brandstetter, H.(2015) Angew Chem Int Ed Engl 54: 2917-2921
- PubMed: 25630877
- DOI: https://doi.org/10.1002/anie.201409135
- Primary Citation of Related Structures:
4N6L, 4N6M, 4N6N, 4N6O - PubMed Abstract:
Peptide ligases expand the repertoire of genetically encoded protein architectures by synthesizing new peptide bonds, energetically driven by ATP or NTPs. Here, we report the discovery of a genuine ligase activity in human legumain (AEP) which has important roles in immunity and tumor progression that were believed to be due to its established cysteine protease activity. Defying dogma, the ligase reaction is independent of the catalytic cysteine but exploits an endogenous energy reservoir that results from the conversion of a conserved aspartate to a metastable aspartimide. Legumain's dual protease-ligase activities are pH- and thus localization controlled, dominating at acidic and neutral pH, respectively. Their relevance includes reversible on-off switching of cystatin inhibitors and enzyme (in)activation, and may affect the generation of three-dimensional MHC epitopes. The aspartate-aspartimide (succinimide) pair represents a new paradigm of coupling endergonic reactions in ATP-scarce environments.
Organizational Affiliation:
Department of Molecular Biology, University of Salzburg, 5020 Salzburg (Austria).