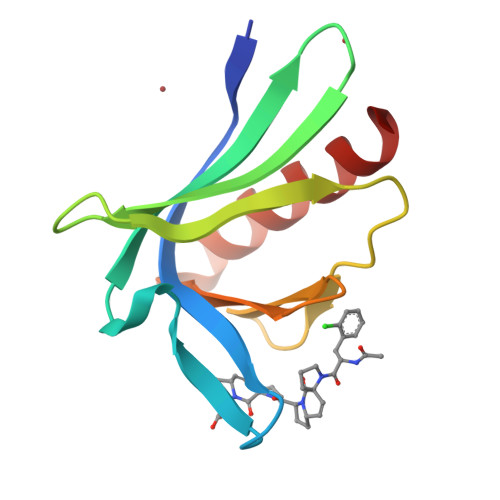
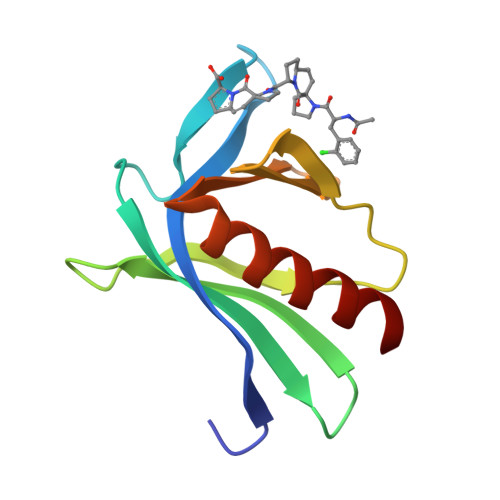
A modular toolkit to inhibit proline-rich motif-mediated protein-protein interactions.
Opitz, R., Muller, M., Reuter, C., Barone, M., Soicke, A., Roske, Y., Piotukh, K., Huy, P., Beerbaum, M., Wiesner, B., Beyermann, M., Schmieder, P., Freund, C., Volkmer, R., Oschkinat, H., Schmalz, H.G., Kuhne, R.(2015) Proc Natl Acad Sci U S A 112: 5011-5016
- PubMed: 25848013
- DOI: https://doi.org/10.1073/pnas.1422054112
- Primary Citation of Related Structures:
4MY6 - PubMed Abstract:
Small-molecule competitors of protein-protein interactions are urgently needed for functional analysis of large-scale genomics and proteomics data. Particularly abundant, yet so far undruggable, targets include domains specialized in recognizing proline-rich segments, including Src-homology 3 (SH3), WW, GYF, and Drosophila enabled (Ena)/vasodilator-stimulated phosphoprotein (VASP) homology 1 (EVH1) domains. Here, we present a modular strategy to obtain an extendable toolkit of chemical fragments (ProMs) designed to replace pairs of conserved prolines in recognition motifs. As proof-of-principle, we developed a small, selective, peptidomimetic inhibitor of Ena/VASP EVH1 domain interactions. Highly invasive MDA MB 231 breast-cancer cells treated with this ligand showed displacement of VASP from focal adhesions, as well as from the front of lamellipodia, and strongly reduced cell invasion. General applicability of our strategy is illustrated by the design of an ErbB4-derived ligand containing two ProM-1 fragments, targeting the yes-associated protein 1 (YAP1)-WW domain with a fivefold higher affinity.
Organizational Affiliation:
Leibniz-Institut für Molekulare Pharmakologie, 13125 Berlin, Germany;