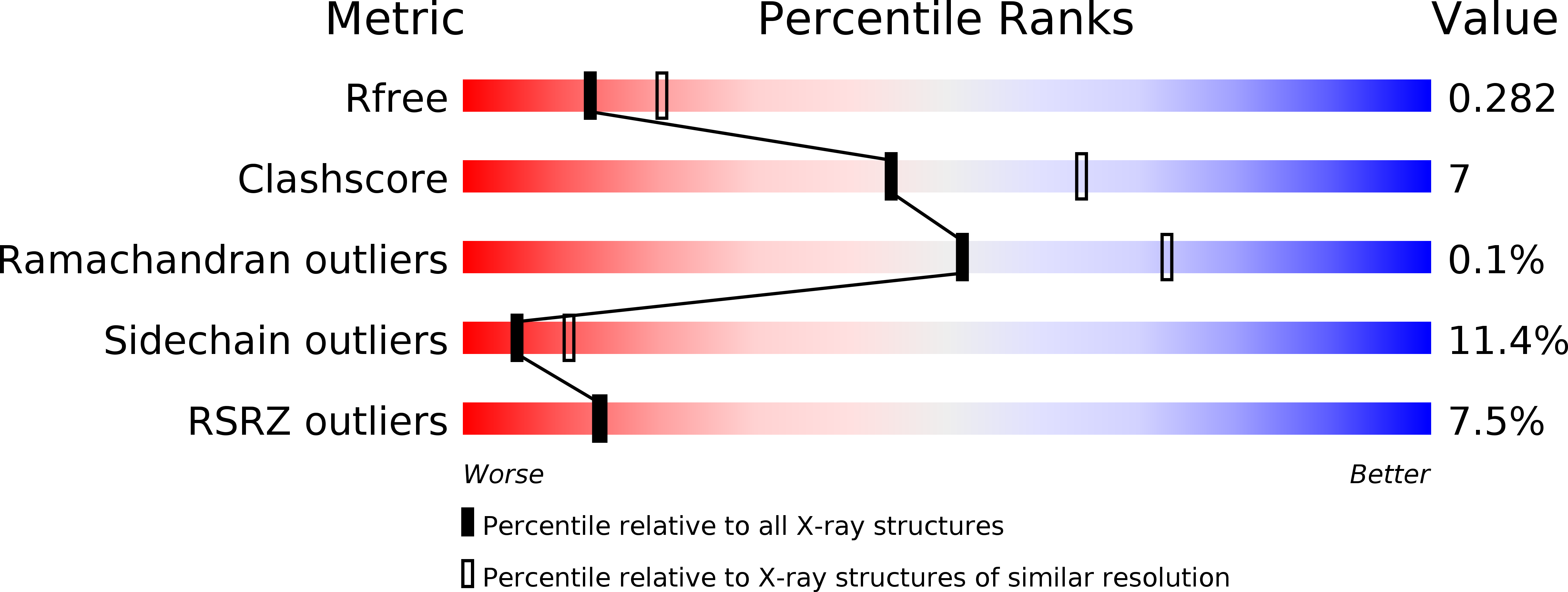
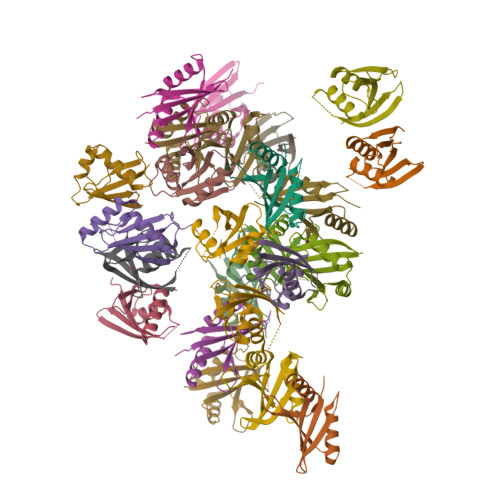
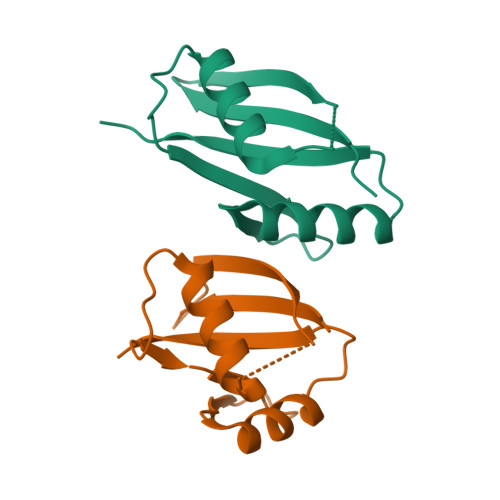
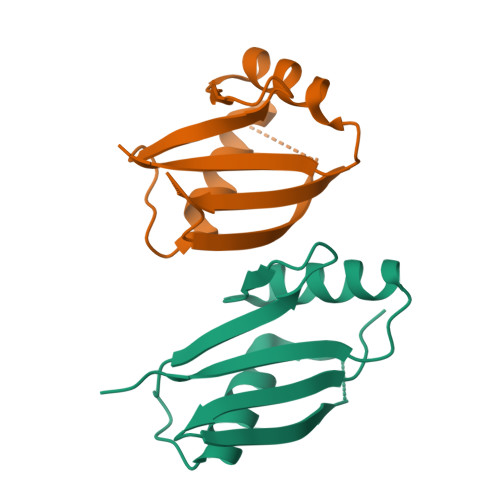
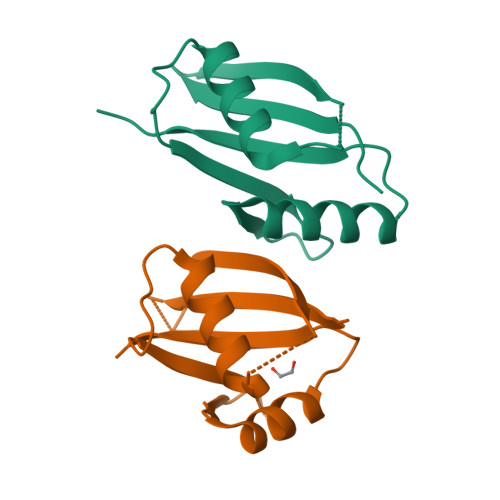
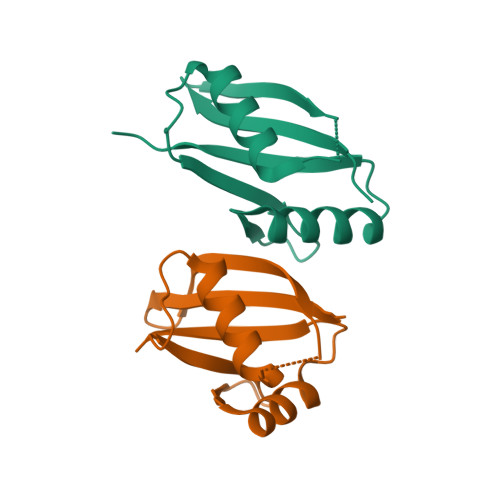
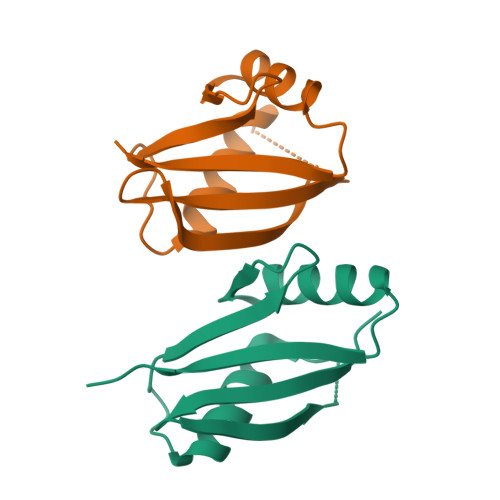
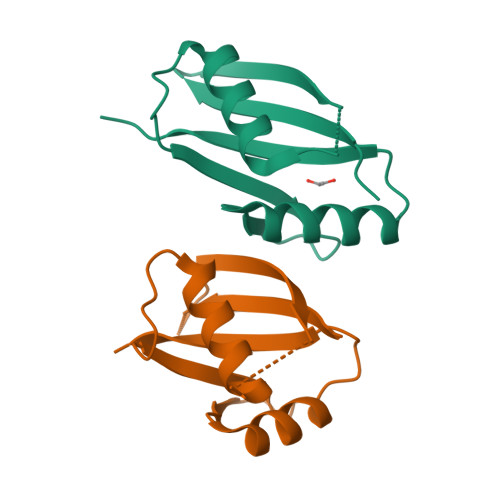
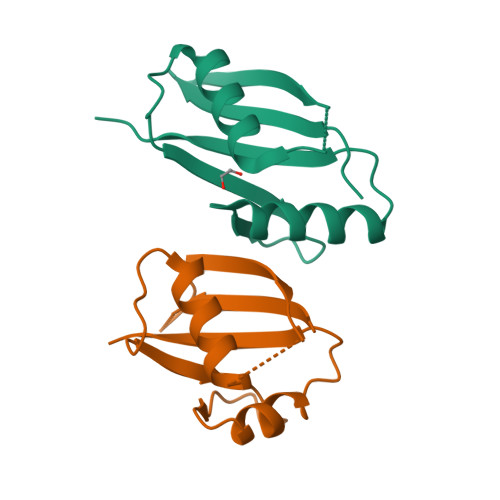
Structural and biochemical insights into the homotypic PB1-PB1 complex between PKC zeta and p62
Ren, J., Wang, J., Wang, Z.X., Wu, J.W.(2014) Sci China Life Sci 57: 69-80
- PubMed: 24369353
- DOI: https://doi.org/10.1007/s11427-013-4592-z
- Primary Citation of Related Structures:
4MJS - PubMed Abstract:
The atypical PKC isoforms (ζ and ı) play essential roles in regulating various cellular processes. Both the hetero-interaction between PKCζ and p62 through their N-terminal PB1 domains and the homo-oligomerization of p62 via its PB1 domain are critical for the activation of NF-κB signaling; however, the molecular mechanisms concerning the formation and regulation of these homotypic complexes remain unclear. Here we determined the crystal structure of PKCζ-PB1 in complex with a monomeric p62-PB1 mutant, where the massive electrostatic interactions between the acidic OPCA motif of PKCζ-PB1 and the basic surface of p62-PB1, as well as additional hydrogen bonds, ensure the formation of a stable and specific complex. The PKCζ-p62 interaction is interfered with the modification of a specific Cys of PKCζ by the antiarthritis drug aurothiomalate, though all four cysteine residues in the PKCζ-PB1 domain can be modified in in vitro assay. In addition, detailed structural and biochemical analyses demonstrate that the PB1 domains of aPKCs belong to the type I group, which can depolymerize the high-molecular-weight p62 aggregates into homo-oligomers of lower order. These data together unravel the molecular mechanisms of the homo-or hetero-interactions between p62 and PKCζ and provide the basis for designing inhibitors of NF-κB signaling.
Organizational Affiliation:
MOE Key Laboratory of Protein Science and Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, 100084, China.