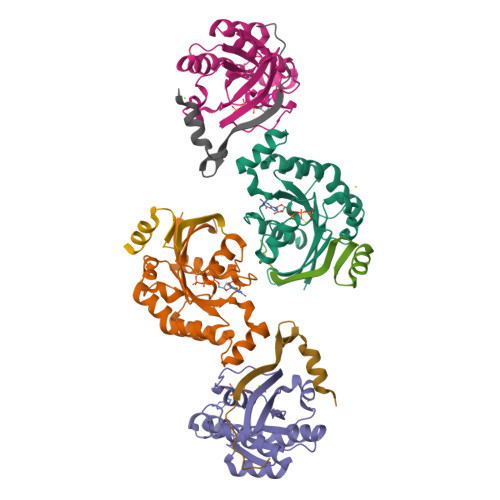
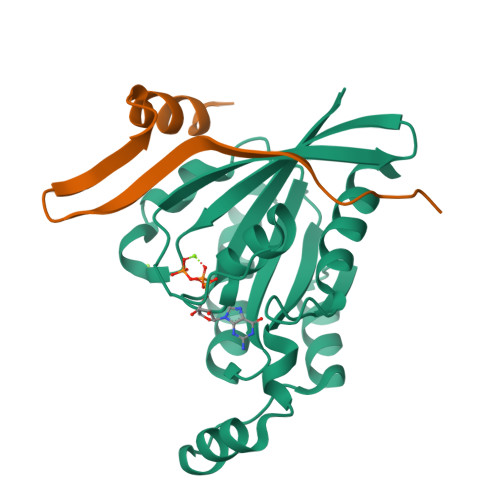
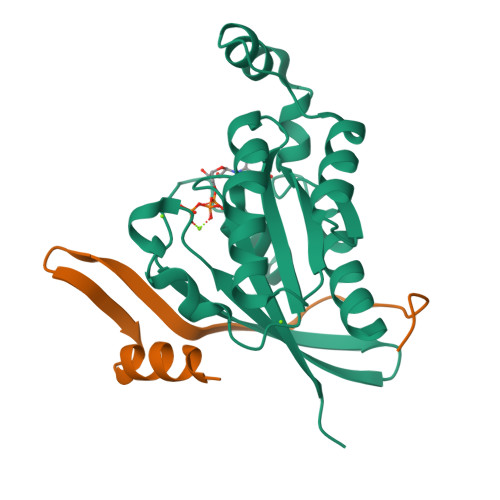
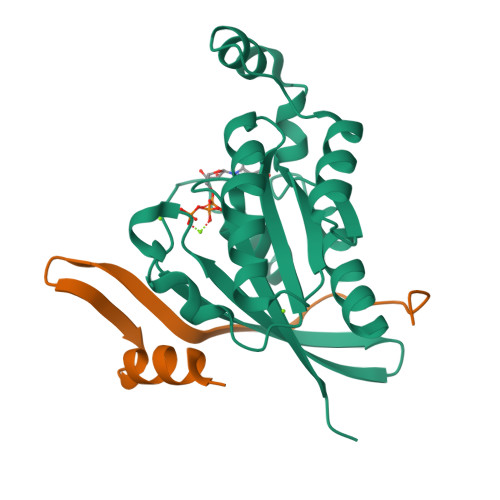
Entamoeba histolytica RacC Selectively Engages p21-Activated Kinase Effectors.
Bosch, D.E., Siderovski, D.P.(2015) Biochemistry 54: 404-412
- PubMed: 25529118
- DOI: https://doi.org/10.1021/bi501226f
- Primary Citation of Related Structures:
4MIT - PubMed Abstract:
Rho family GTPases modulate actin cytoskeleton dynamics by signaling through multiple effectors, including the p21-activated kinases (PAKs). The intestinal parasite Entamoeba histolytica expresses ∼20 Rho family GTPases and seven isoforms of PAK, two of which have been implicated in pathogenesis-related processes such as amoebic motility and invasion and host cell phagocytosis. Here, we describe two previously unstudied PAK isoforms, EhPAK4 and EhPAK5, as highly specific effectors of EhRacC. A structural model based on 2.35 Å X-ray crystallographic data of a complex between EhRacC(Q65L)·GTP and the EhPAK4 p21 binding domain (PBD) reveals a fairly well-conserved Rho/effector interface despite deviation of the PBD α-helix. A structural comparison with EhRho1 in complex with EhFormin1 suggests likely determinants of Rho family GTPase signaling specificity in E. histolytica. These findings suggest a high degree of Rho family GTPase diversity and specificity in the single-cell parasite E. histolytica. Because PAKs regulate pathogenesis-related processes in E. histolytica, they may be valid pharmacologic targets for anti-amoebiasis drugs.
Organizational Affiliation:
Department of Pharmacology, The University of North Carolina , Chapel Hill, North Carolina 27514, United States.