GluK1 antagonists from 6-(tetrazolyl)phenyl decahydroisoquinoline derivatives: in vitro profile and in vivo analgesic efficacy.
Martinez-Perez, J.A., Iyengar, S., Shannon, H.E., Bleakman, D., Alt, A., Clawson, D.K., Arnold, B.M., Bell, M.G., Bleisch, T.J., Castano, A.M., Del Prado, M., Dominguez, E., Escribano, A.M., Filla, S.A., Ho, K.H., Hudziak, K.J., Jones, C.K., Mateo, A., Mathes, B.M., Mattiuz, E.L., Ogden, A.M., Simmons, R.M., Stack, D.R., Stratford, R.E., Winter, M.A., Wu, Z., Ornstein, P.L.(2013) Bioorg Med Chem Lett 23: 6463-6466
- PubMed: 24140446
- DOI: https://doi.org/10.1016/j.bmcl.2013.09.045
- Primary Citation of Related Structures:
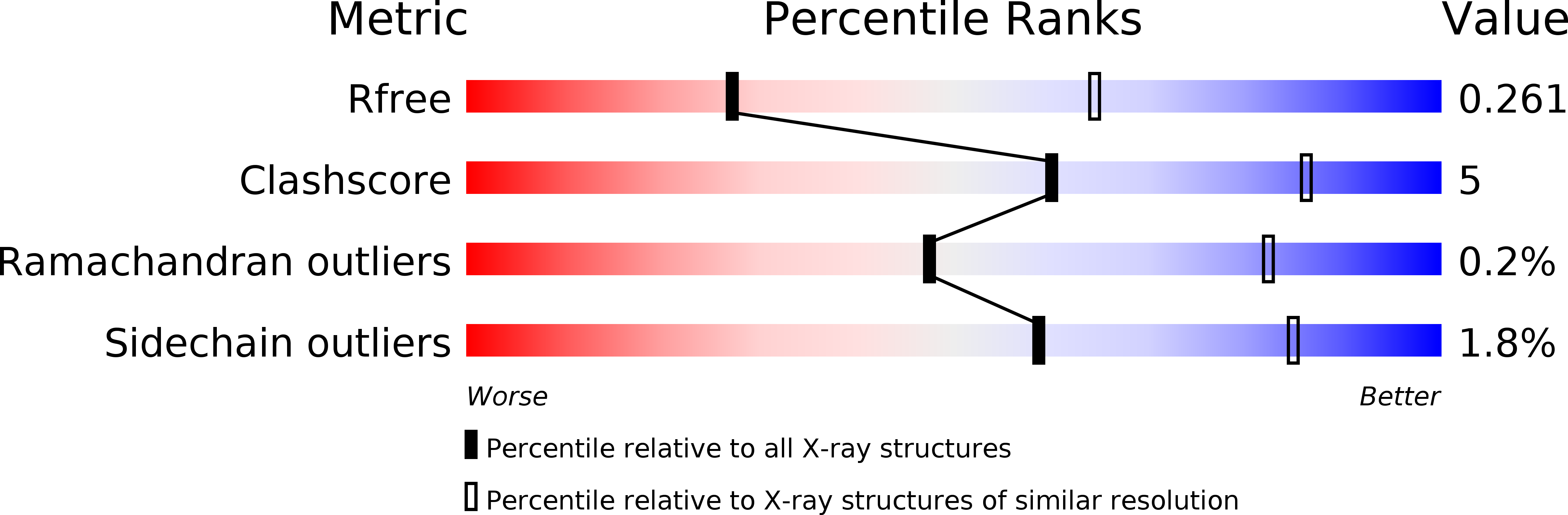
4MF3 - PubMed Abstract:
We have explored the decahydroisoquinoline scaffold, bearing a phenyl tetrazole, as GluK1 antagonists with potential as oral analgesics. We have established the optimal linker atom between decahydroisoquinoline and phenyl rings and demonstrated an improvement of both the affinity for the GluK1 receptor and the selectivity against the related GluA2 receptor with proper phenyl substitution. In this Letter, we also disclose in vivo data that led to the discovery of LY545694·HCl, a compound with oral efficacy in two persistent pain models.
Organizational Affiliation:
Centro de Investigación Lilly, Avda. de la Industria, 30, 28108 Alcobendas, Madrid, Spain. Electronic address: Martinez-Perez_Jose_A@Lilly.com.