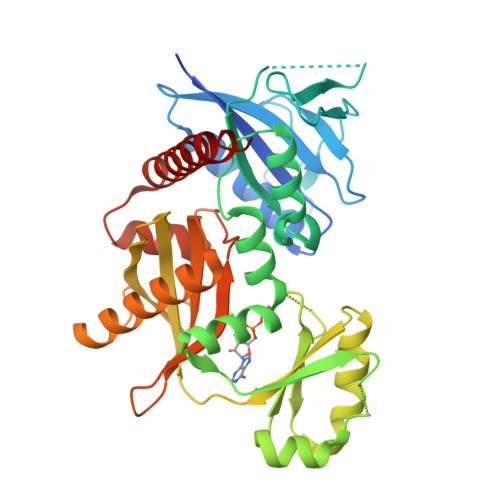
Crystal structures of d-alanine-d-alanine ligase from Xanthomonas oryzae pv. oryzae alone and in complex with nucleotides
Doan, T.N.N., Kim, J.K., Ngo, H.P.T., Tran, H.T., Cha, S.S., Chung, K.M., Huynh, K.H., Ahn, Y.J., Kang, L.W.(2014) Arch Biochem Biophys 545C: 92-99
- PubMed: 24440607
- DOI: https://doi.org/10.1016/j.abb.2014.01.009
- Primary Citation of Related Structures:
4L1K, 4ME6 - PubMed Abstract:
D-Alanine-D-alanine ligase (DDL) catalyzes the biosynthesis of d-alanyl-d-alanine, an essential bacterial peptidoglycan precursor, and is an important drug target for the development of antibacterials. We determined four different crystal structures of DDL from Xanthomonas oryzae pv. oryzae (Xoo) causing Bacteria Blight (BB), which include apo, ADP-bound, ATP-bound, and AMPPNP-bound structures at the resolution between 2.3 and 2.0 Å. Similarly with other DDLs, the active site of XoDDL is formed by three loops from three domains at the center of enzyme. Compared with d-alanyl-d-alanine and ATP-bound TtDDL structure, the γ-phosphate of ATP in XoDDL structure was shifted outside toward solution. We swapped the ω-loop (loop3) of XoDDL with those of Escherichia coli and Helicobacter pylori DDLs, and measured the enzymatic kinetics of wild-type XoDDL and two mutant XoDDLs with the swapped ω-loops. Results showed that the direct interactions between ω-loop and other two loops are essential for the active ATP conformation for D-ala-phosphate formation.
Organizational Affiliation:
Department of Advanced Technology Fusion, Konkuk University, Hwayang dong, Gwangjin-gu, Seoul 143-01, Republic of Korea.