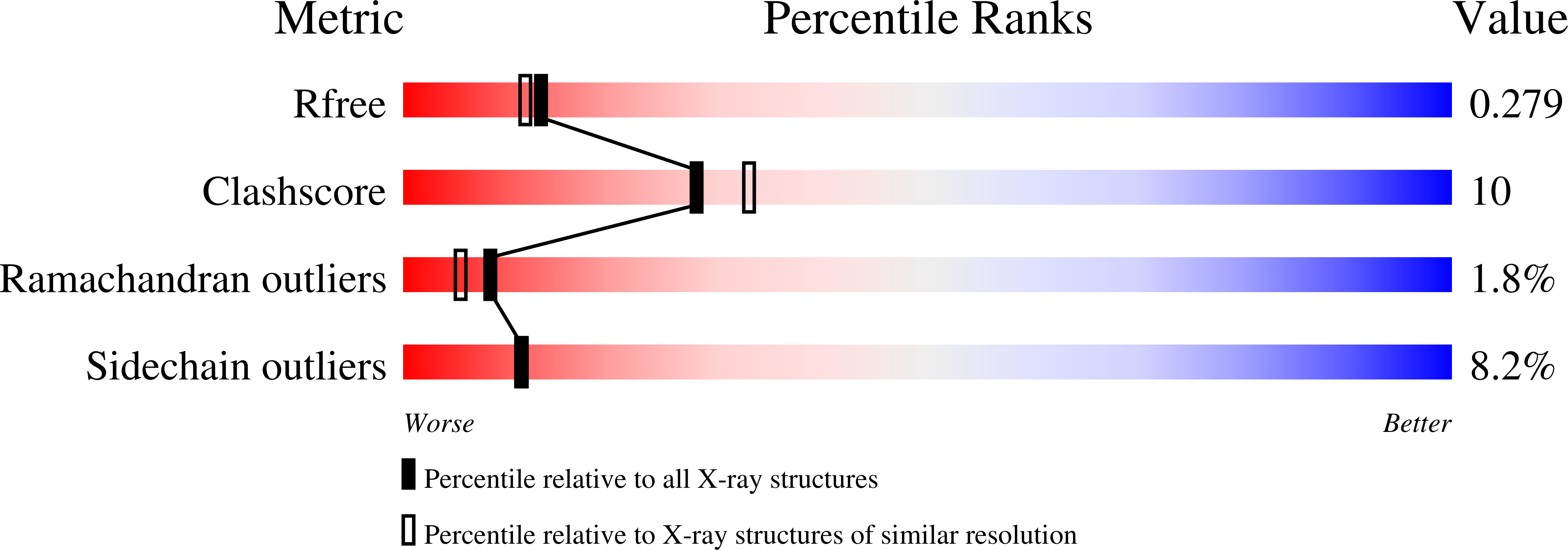Characterization of Solanum tuberosum Multicystatin and the Significance of Core Domains.
Green, A.R., Nissen, M.S., Kumar, G.N., Knowles, N.R., Kang, C.(2013) Plant Cell 25: 5043-5052
- PubMed: 24363310
- DOI: https://doi.org/10.1105/tpc.113.121004
- Primary Citation of Related Structures:
4LZI - PubMed Abstract:
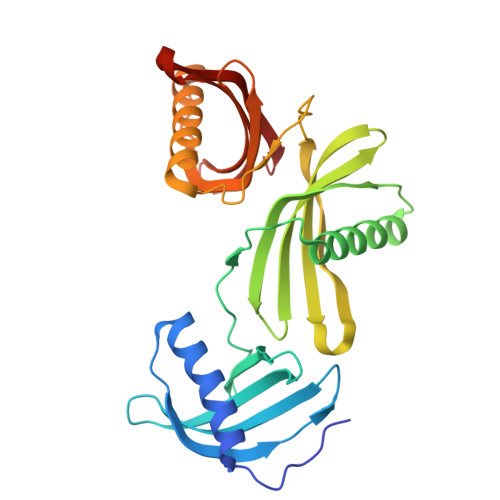
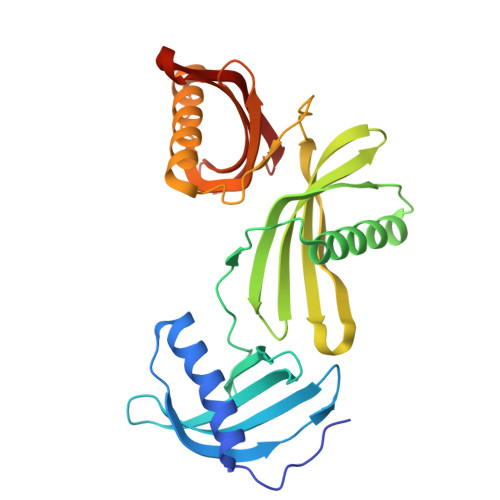
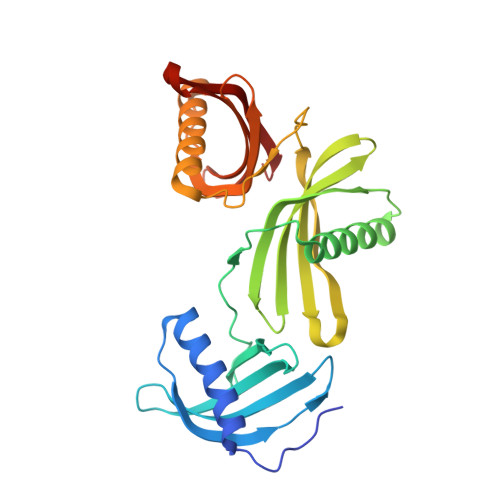
Potato (Solanum tuberosum) multicystatin (PMC) is a unique cystatin composed of eight repeating units, each capable of inhibiting cysteine proteases. PMC is a composite of several cystatins linked by trypsin-sensitive (serine protease) domains and undergoes transitions between soluble and crystalline forms. However, the significance and the regulatory mechanism or mechanisms governing these transitions are not clearly established. Here, we report the 2.2-Å crystal structure of the trypsin-resistant PMC core consisting of the fifth, sixth, and seventh domains. The observed interdomain interaction explains PMC's resistance to trypsin and pH-dependent solubility/aggregation. Under acidic pH, weakening of the interdomain interactions exposes individual domains, resulting in not only depolymerization of the crystalline form but also exposure of cystatin domains for inhibition of cysteine proteases. This in turn allows serine protease-mediated fragmentation of PMC, producing ∼ 10-kD domains with intact inhibitory capacity and faster diffusion, thus enhancing PMC's inhibitory ability toward cysteine proteases. The crystal structure, light-scattering experiments, isothermal titration calorimetry, and site-directed mutagenesis confirmed the critical role of pH and N-terminal residues in these dynamic transitions between monomer/polymer of PMC. Our data support a notion that the pH-dependent structural regulation of PMC has defense-related implications in tuber physiology via its ability to regulate protein catabolism.
Organizational Affiliation:
School of Molecular Biosciences, Washington State University, Pullman, Washington 99164.