Cytochrome c6B of Synechococcus sp. WH 8102 - Crystal structure and basic properties of novel c6-like family representative.
Zatwarnicki, P., Barciszewski, J., Krzywda, S., Jaskolski, M., Kolesinski, P., Szczepaniak, A.(2014) Biochem Biophys Res Commun 443: 1131-1135
- PubMed: 24216109
- DOI: https://doi.org/10.1016/j.bbrc.2013.10.167
- Primary Citation of Related Structures:
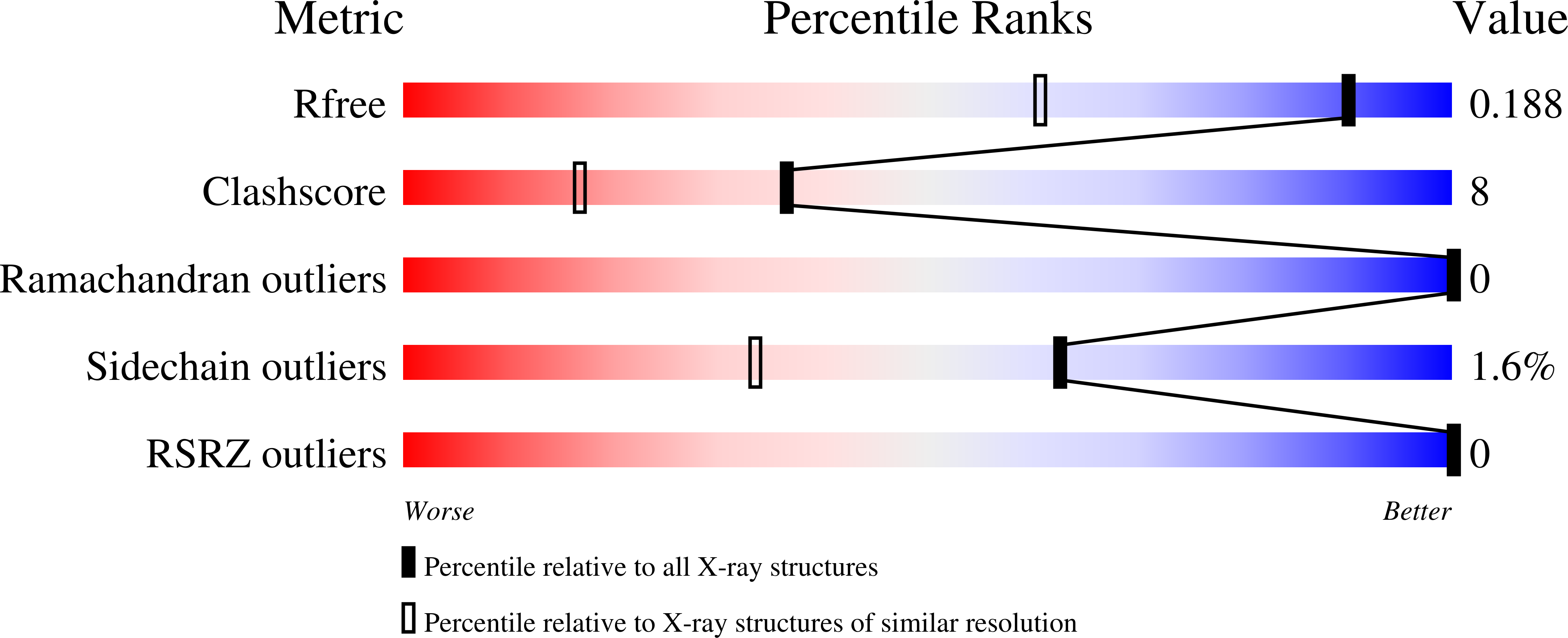
4KMG - PubMed Abstract:
Cytochromes c are soluble electron carriers of relatively low molecular weight, containing single heme moiety. In cyanobacteria cytochrome c6 participates in electron transfer from cytochrome b6f complex to photosystem I. Recent phylogenetic analysis revealed the existence of a few families of proteins homologous to the previously mentioned. Cytochrome c6A from Arabidopsis thaliana was identified as a protein responsible for disulfide bond formation in response to intracellular redox state changes and c550 is well known element of photosystem II. However, function of cytochromes marked as c6B, c6C and cM as well as the physiological process in which they take a part still remain unidentified. Here we present the first structural and biophysical analysis of cytochrome from the c6B family from mesophilic cyanobacteria Synechococcus sp. WH 8102. Purified protein was crystallized and its structure was refined at 1.4 Å resolution. Overall architecture of this polypeptide resembles typical I-class cytochromes c. The main features, that distinguish described protein from cytochrome c6, are slightly red-shifted α band of UV-Vis spectrum as well as relatively low midpoint potential (113.2±2.2 mV). Although, physiological function of cytochrome c6B has yet to be determined its properties probably exclude the participation of this protein in electron trafficking between b6f complex and photosystem I.
Organizational Affiliation:
Department of Biophysics, Faculty of Biotechnology, University of Wroclaw, F. Joliot Curie 14a, 50-383 Wroclaw, Poland.