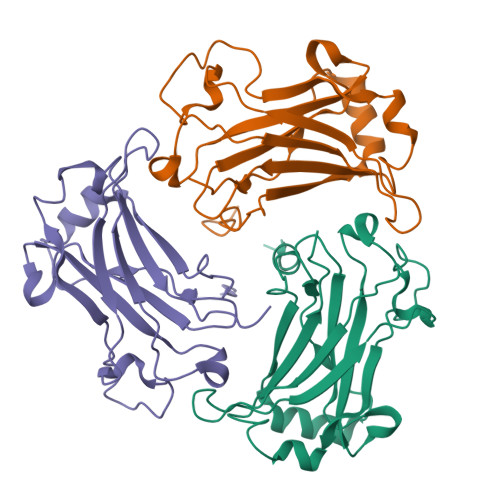
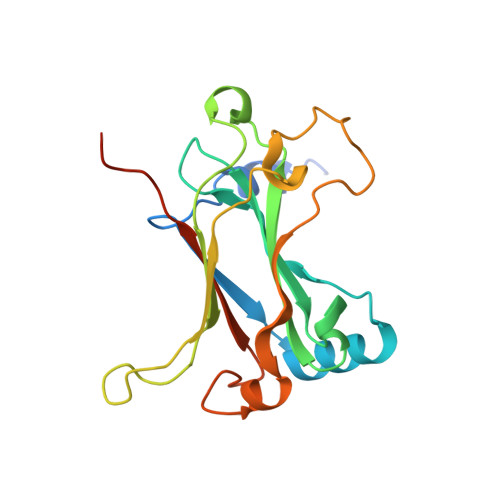
Structure of the TRAF4 TRAF domain with a coiled-coil domain and its implications for the TRAF4 signalling pathway.
Yoon, J.H., Cho, Y.J., Park, H.H.(2014) Acta Crystallogr D Biol Crystallogr 70: 2-10
- PubMed: 24419373
- DOI: https://doi.org/10.1107/S139900471302333X
- Primary Citation of Related Structures:
4K8U - PubMed Abstract:
The TNF receptor-associated factor (TRAF) proteins are structurally similar scaffold proteins that mediate between members of the TNF receptor (TNFR) family and downstream effector molecules such as kinases in the immune signalling pathway. Seven TRAFs have been identified, including TRAF4, which is a unique member that participates in many ontogenic processes, including nerve-system development. TRAFs commonly contain the TRAF domain, which mediates interaction with target receptors and effectors. As a first step towards elucidating the molecular mechanisms of the TRAF4-mediated signalling pathway, the first crystal structure of the human TRAF4 TRAF domain with a coiled-coil domain is reported at 2.3 Å resolution.
Organizational Affiliation:
School of Biotechnology and Graduate School of Biochemistry, Yeungnam University, Gyeongsan, Republic of Korea.