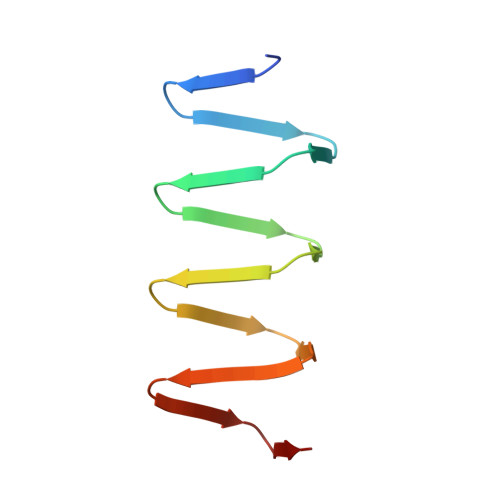
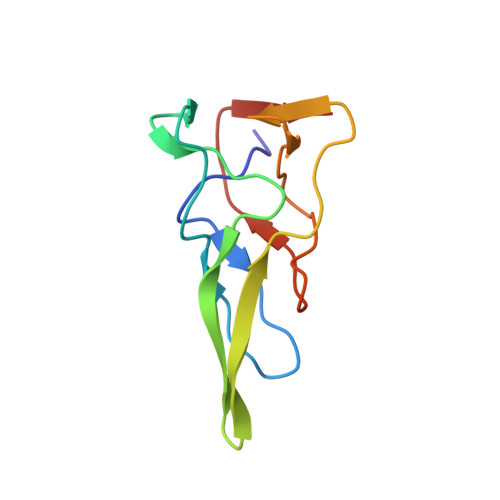
PAAR-repeat proteins sharpen and diversify the type VI secretion system spike.
Shneider, M.M., Buth, S.A., Ho, B.T., Basler, M., Mekalanos, J.J., Leiman, P.G.(2013) Nature 500: 350-353
- PubMed: 23925114
- DOI: https://doi.org/10.1038/nature12453
- Primary Citation of Related Structures:
4JIV, 4JIW - PubMed Abstract:
The bacterial type VI secretion system (T6SS) is a large multicomponent, dynamic macromolecular machine that has an important role in the ecology of many Gram-negative bacteria. T6SS is responsible for translocation of a wide range of toxic effector molecules, allowing predatory cells to kill both prokaryotic as well as eukaryotic prey cells. The T6SS organelle is functionally analogous to contractile tails of bacteriophages and is thought to attack cells by initially penetrating them with a trimeric protein complex called the VgrG spike. Neither the exact protein composition of the T6SS organelle nor the mechanisms of effector selection and delivery are known. Here we report that proteins from the PAAR (proline-alanine-alanine-arginine) repeat superfamily form a sharp conical extension on the VgrG spike, which is further involved in attaching effector domains to the spike. The crystal structures of two PAAR-repeat proteins bound to VgrG-like partners show that these proteins sharpen the tip of the T6SS spike complex. We demonstrate that PAAR proteins are essential for T6SS-mediated secretion and target cell killing by Vibrio cholerae and Acinetobacter baylyi. Our results indicate a new model of the T6SS organelle in which the VgrG-PAAR spike complex is decorated with multiple effectors that are delivered simultaneously into target cells in a single contraction-driven translocation event.
- École Polytechnique Fédérale de Lausanne (EPFL), BSP-415, 1015 Lausanne, Switzerland.
Organizational Affiliation: