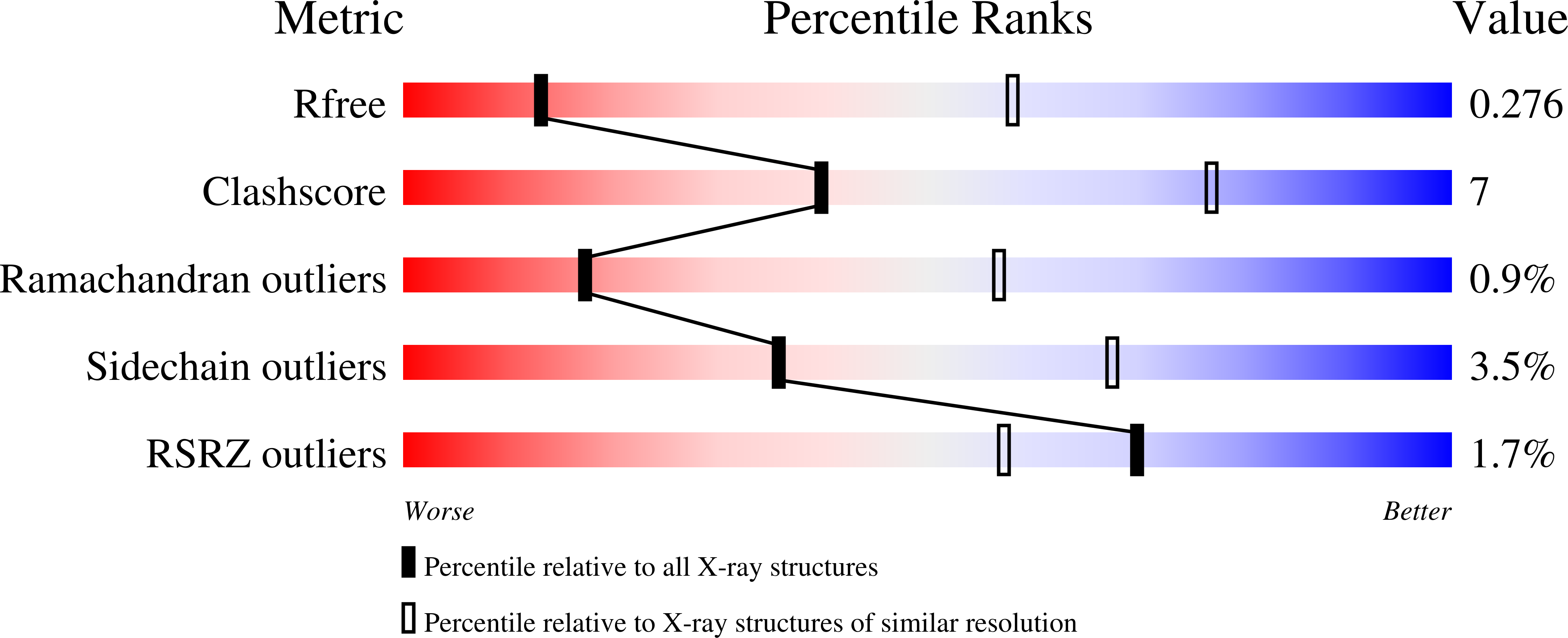
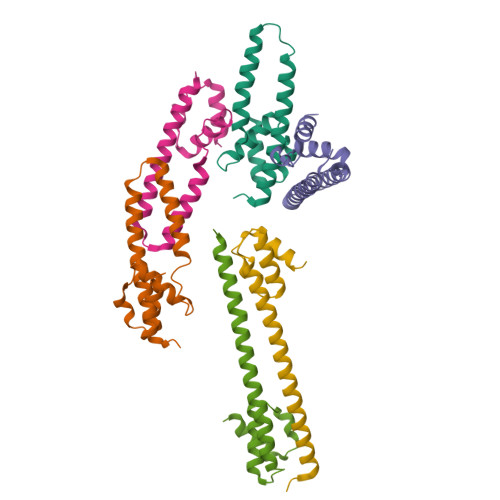
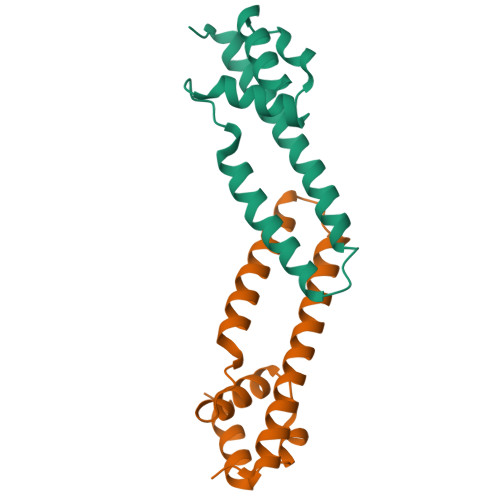
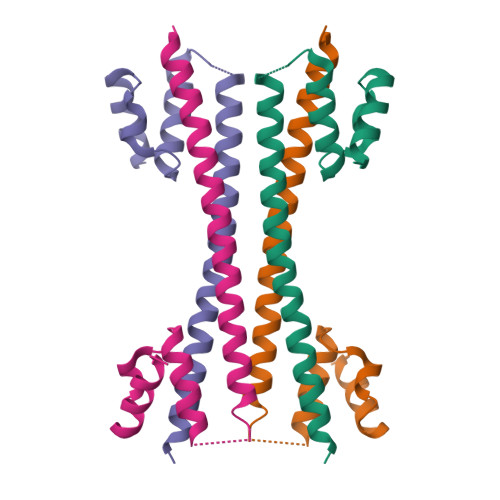
Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria.
Sutter, M., Wilson, A., Leverenz, R.L., Lopez-Igual, R., Thurotte, A., Salmeen, A.E., Kirilovsky, D., Kerfeld, C.A.(2013) Proc Natl Acad Sci U S A 110: 10022-10027
- PubMed: 23716688
- DOI: https://doi.org/10.1073/pnas.1303673110
- Primary Citation of Related Structures:
4JDQ, 4JDX - PubMed Abstract:
Photosynthetic reaction centers are sensitive to high light conditions, which can cause damage because of the formation of reactive oxygen species. To prevent high-light induced damage, cyanobacteria have developed photoprotective mechanisms. One involves a photoactive carotenoid protein that decreases the transfer of excess energy to the reaction centers. This protein, the orange carotenoid protein (OCP), is present in most cyanobacterial strains; it is activated by high light conditions and able to dissipate excess energy at the site of the light-harvesting antennae, the phycobilisomes. Restoration of normal antenna capacity involves the fluorescence recovery protein (FRP). The FRP acts to dissociate the OCP from the phycobilisomes by accelerating the conversion of the active red OCP to the inactive orange form. We have determined the 3D crystal structure of the FRP at 2.5 Å resolution. Remarkably, the FRP is found in two very different conformational and oligomeric states in the same crystal. Based on amino acid conservation analysis, activity assays of FRP mutants, FRP:OCP docking simulations, and coimmunoprecipitation experiments, we conclude that the dimer is the active form. The second form, a tetramer, may be an inactive form of FRP. In addition, we have identified a surface patch of highly conserved residues and shown that those residues are essential to FRP activity.
Organizational Affiliation:
US Department of Energy, Joint Genome Institute, Walnut Creek, CA 94598, USA.