Characterization of PUD-1 and PUD-2, two proteins up-regulated in a long-lived daf-2 mutant.
Ding, Y.H., Du, Y.G., Luo, S., Li, Y.X., Li, T.M., Yoshina, S., Wang, X., Klage, K., Mitani, S., Ye, K., Dong, M.Q.(2013) PLoS One 8: e67158-e67158
- PubMed: 23799143
- DOI: https://doi.org/10.1371/journal.pone.0067158
- Primary Citation of Related Structures:
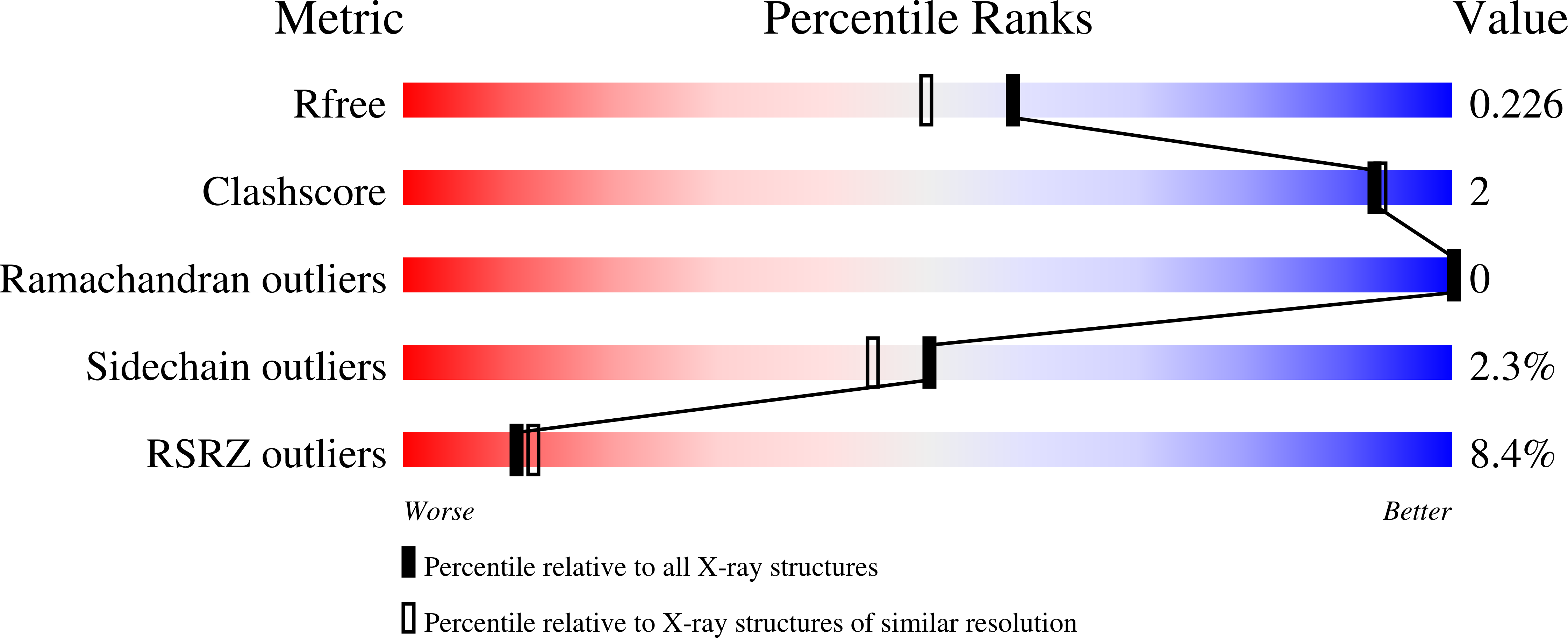
4JDE - PubMed Abstract:
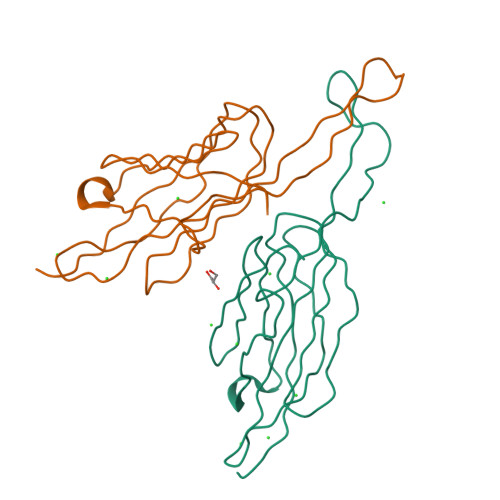
C. elegans PUD-1 and PUD-2, two proteins up-regulated in daf-2(loss-of-function) (PUD), are homologous 17-kD proteins with a large abundance increase in long-lived daf-2 mutant animals of reduced insulin signaling. In this study, we show that both PUD-1 and PUD-2 are abundantly expressed in the intestine and hypodermis, and form a heterodimer. We have solved their crystal structure to 1.9-Å resolution and found that both proteins adopt similar β-sandwich folds in the V-shaped dimer. In contrast, their homologs PUD-3, PUD-4, PUDL-1 and PUDL-2 are all monomeric proteins with distinct expression patterns in C. elegans. Thus, the PUD-1/PUD-2 heterodimer probably has a function distinct from their family members. Neither overexpression nor deletion of pud-1 and pud-2 affected the lifespan of WT or daf-2 mutant animals, suggesting that their induction in daf-2 worms does not contribute to longevity. Curiously, deletion of pud-1 and pud-2 was associated with a protective effect against paralysis induced by the amyloid β-peptide (1-42), which further enhanced the protection conferred by daf-2(RNAi) against Aβ.
Organizational Affiliation:
National Institute of Biological Sciences, Beijing, Beijing, China.