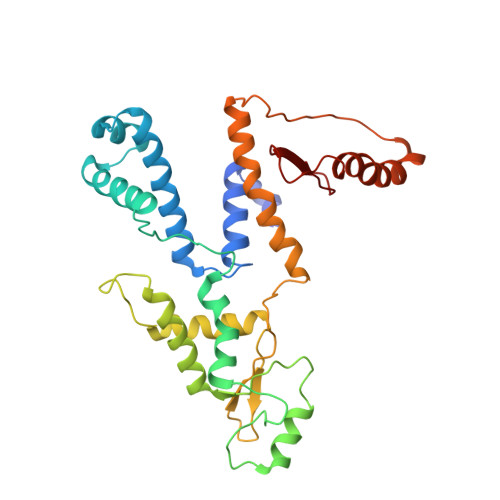
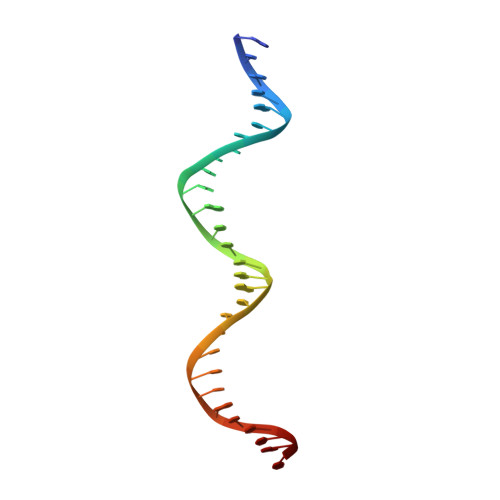
Structures of complexes comprised of Fischerella transcription factor HetR with Anabaena DNA targets.
Kim, Y., Ye, Z., Joachimiak, G., Videau, P., Young, J., Hurd, K., Callahan, S.M., Gornicki, P., Zhao, J., Haselkorn, R., Joachimiak, A.(2013) Proc Natl Acad Sci U S A 110: E1716-E1723
- PubMed: 23610410
- DOI: https://doi.org/10.1073/pnas.1305971110
- Primary Citation of Related Structures:
4IZZ, 4J00, 4J01 - PubMed Abstract:
HetR is an essential regulator of heterocyst development in cyanobacteria. Many mutations in HetR render Anabaena incapable of nitrogen fixation. The protein binds to a DNA palindrome upstream of hetP and other genes. We have determined the crystal structures of HetR complexed with palindromic DNA targets, 21, 23, and 29 bp at 2.50-, 3.00-, and 3.25-Å resolution, respectively. The highest-resolution structure shows fine details of specific protein-DNA interactions. The lower-resolution structures with longer DNA duplexes have similar interaction patterns and show how the flap domains interact with DNA in a sequence nonspecific fashion. Fifteen of 15 protein-DNA contacts predicted on the basis of the structure were confirmed by single amino acid mutations that abolished binding in vitro and complementation in vivo. A striking feature of the structure is the association of glutamate 71 from each subunit of the HetR dimer with three successive cytosines in each arm of the palindromic target, a feature that is conserved among all known heterocyst-forming cyanobacteria sequenced to date.
Organizational Affiliation:
Midwest Center for Structural Genomics and Structural Biology Center, Biosciences, Argonne National Laboratory, Argonne, IL 60439, USA.