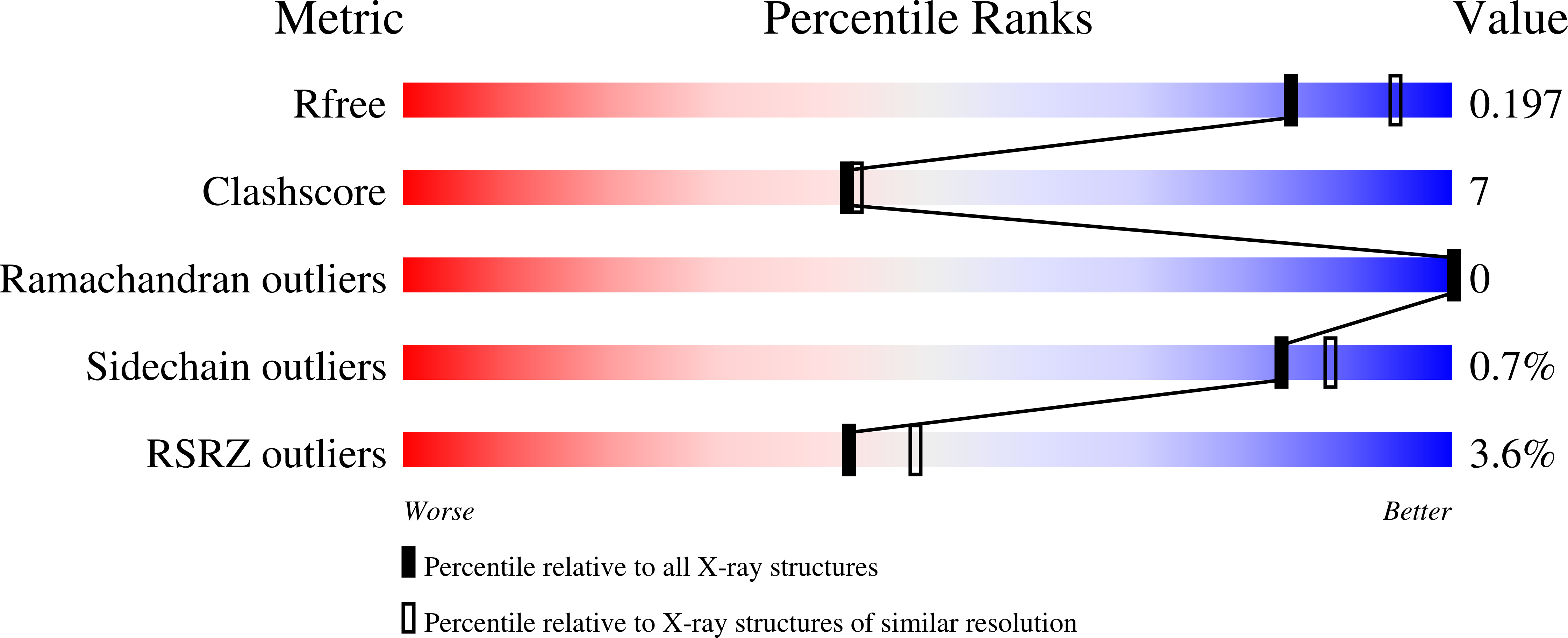
Mechanistic Characterization of the Tetraacyldisaccharide-1-phosphate 4'-Kinase LpxK Involved in Lipid A Biosynthesis.
Emptage, R.P., Pemble, C.W., York, J.D., Raetz, C.R., Zhou, P.(2013) Biochemistry 52: 2280-2290
- PubMed: 23464738
- DOI: https://doi.org/10.1021/bi400097z
- Primary Citation of Related Structures:
4ITL, 4ITM, 4ITN - PubMed Abstract:
The sixth step in the lipid A biosynthetic pathway involves phosphorylation of the tetraacyldisaccharide-1-phosphate (DSMP) intermediate by the cytosol-facing inner membrane kinase LpxK, a member of the P-loop-containing nucleoside triphosphate (NTP) hydrolase superfamily. We report the kinetic characterization of LpxK from Aquifex aeolicus and the crystal structures of LpxK in complex with ATP in a precatalytic binding state, the ATP analogue AMP-PCP in the closed catalytically competent conformation, and a chloride anion revealing an inhibitory conformation of the nucleotide-binding P-loop. We demonstrate that LpxK activity in vitro requires the presence of a detergent micelle and formation of a ternary LpxK-ATP/Mg(2+)-DSMP complex. Using steady-state kinetics, we have identified crucial active site residues, leading to the proposal that the interaction of D99 with H261 acts to increase the pKa of the imidazole moiety, which in turn serves as the catalytic base to deprotonate the 4'-hydroxyl of the DSMP substrate. The fact that an analogous mechanism has not yet been observed for other P-loop kinases highlights LpxK as a distinct member of the P-loop kinase family, a notion that is also reflected through its localization at the membrane, lipid substrate, and overall structure.
Organizational Affiliation:
Department of Biochemistry, Duke University Medical Center, Durham, NC 27710, USA. ryan.emptage@duke.edu