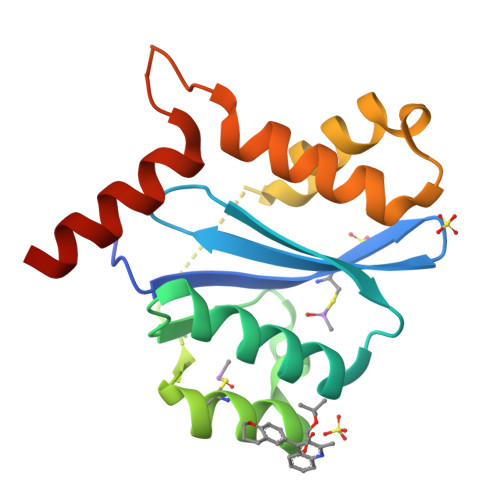
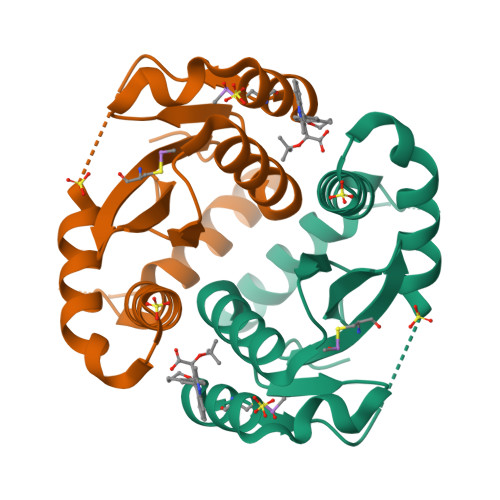
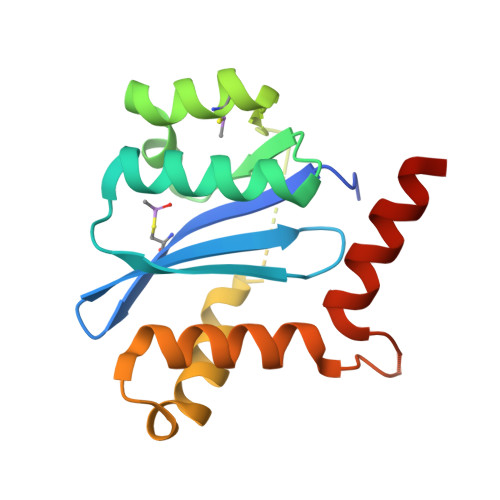
Allosteric integrase inhibitor potency is determined through the inhibition of HIV-1 particle maturation.
Jurado, K.A., Wang, H., Slaughter, A., Feng, L., Kessl, J.J., Koh, Y., Wang, W., Ballandras-Colas, A., Patel, P.A., Fuchs, J.R., Kvaratskhelia, M., Engelman, A.(2013) Proc Natl Acad Sci U S A 110: 8690-8695
- PubMed: 23610442
- DOI: https://doi.org/10.1073/pnas.1300703110
- Primary Citation of Related Structures:
4ID1 - PubMed Abstract:
Integration is essential for HIV-1 replication, and the viral integrase (IN) protein is an important therapeutic target. Allosteric IN inhibitors (ALLINIs) that engage the IN dimer interface at the binding site for the host protein lens epithelium-derived growth factor (LEDGF)/transcriptional coactivator p75 are an emerging class of small molecule antagonists. Consistent with the inhibition of a multivalent drug target, ALLINIs display steep antiviral dose-response curves ex vivo. ALLINIs multimerize IN protein and concordantly block its assembly with viral DNA in vitro, indicating that the disruption of two integration-associated functions, IN catalysis and the IN-LEDGF/p75 interaction, determines the multimode mechanism of ALLINI action. We now demonstrate that ALLINI potency is unexpectedly accounted for during the late phase of HIV-1 replication. The compounds promote virion IN multimerization and, reminiscent of class II IN mutations, block the formation of the electron-dense viral core and inhibit reverse transcription and integration in subsequently infected target cells. Mature virions are recalcitrant to ALLINI treatment, and compound potency during virus production is independent of the level of LEDGF/p75 expression. We conclude that cooperative multimerization of IN by ALLINIs together with the inability for LEDGF/p75 to effectively engage the virus during its egress from cells underscores the multimodal mechanism of ALLINI action. Our results highlight the versatile nature of allosteric inhibitors to primarily inhibit viral replication at a step that is distinct from the catalytic requirement for the target enzyme. The vulnerability of IN to small molecules during the late phase of HIV-1 replication unveils a pharmacological Achilles' heel for exploitation in clinical ALLINI development.
Organizational Affiliation:
Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02215, USA.