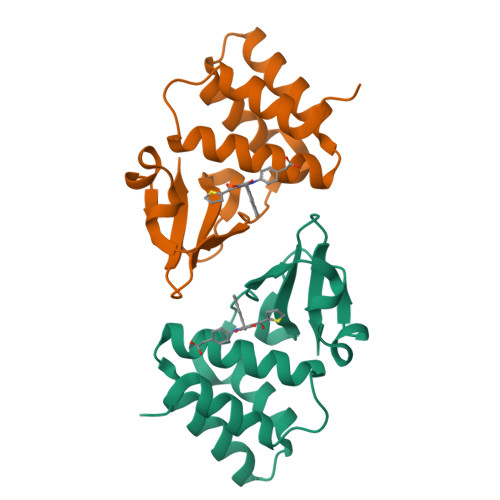
In Silico Derived Small Molecules Bind the Filovirus VP35 Protein and Inhibit Its Polymerase Cofactor Activity.
Brown, C.S., Lee, M.S., Leung, D.W., Wang, T., Xu, W., Luthra, P., Anantpadma, M., Shabman, R.S., Melito, L.M., Macmillan, K.S., Borek, D.M., Otwinowski, Z., Ramanan, P., Stubbs, A.J., Peterson, D.S., Binning, J.M., Tonelli, M., Olson, M.A., Davey, R.A., Ready, J.M., Basler, C.F., Amarasinghe, G.K.(2014) J Mol Biology 426: 2045-2058
- PubMed: 24495995
- DOI: https://doi.org/10.1016/j.jmb.2014.01.010
- Primary Citation of Related Structures:
4IBB, 4IBC, 4IBD, 4IBE, 4IBF, 4IBG, 4IBI, 4IBJ, 4IBK - PubMed Abstract:
The Ebola virus (EBOV) genome only encodes a single viral polypeptide with enzymatic activity, the viral large (L) RNA-dependent RNA polymerase protein. However, currently, there is limited information about the L protein, which has hampered the development of antivirals. Therefore, antifiloviral therapeutic efforts must include additional targets such as protein-protein interfaces. Viral protein 35 (VP35) is multifunctional and plays important roles in viral pathogenesis, including viral mRNA synthesis and replication of the negative-sense RNA viral genome. Previous studies revealed that mutation of key basic residues within the VP35 interferon inhibitory domain (IID) results in significant EBOV attenuation, both in vitro and in vivo. In the current study, we use an experimental pipeline that includes structure-based in silico screening and biochemical and structural characterization, along with medicinal chemistry, to identify and characterize small molecules that target a binding pocket within VP35. NMR mapping experiments and high-resolution x-ray crystal structures show that select small molecules bind to a region of VP35 IID that is important for replication complex formation through interactions with the viral nucleoprotein (NP). We also tested select compounds for their ability to inhibit VP35 IID-NP interactions in vitro as well as VP35 function in a minigenome assay and EBOV replication. These results confirm the ability of compounds identified in this study to inhibit VP35-NP interactions in vitro and to impair viral replication in cell-based assays. These studies provide an initial framework to guide development of antifiloviral compounds against filoviral VP35 proteins.
Organizational Affiliation:
Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO 63110, USA; Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology, Iowa State University, Ames, IA 50011, USA; Biochemistry Undergraduate Program, Iowa State University, Ames, IA 50011, USA.