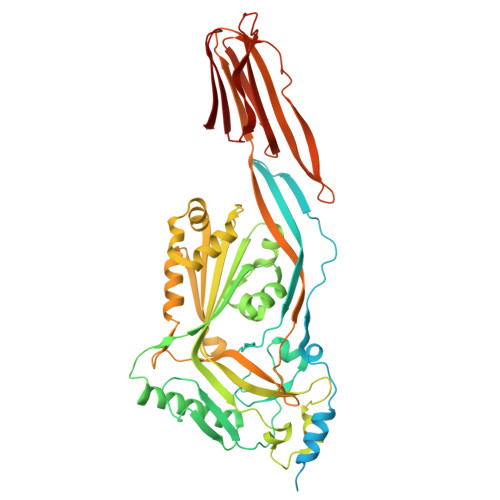
Structural studies of Streptococcus pyogenes streptolysin O provide insights into the early steps of membrane penetration.
Feil, S.C., Ascher, D.B., Kuiper, M.J., Tweten, R.K., Parker, M.W.(2014) J Mol Biology 426: 785-792
- PubMed: 24316049
- DOI: https://doi.org/10.1016/j.jmb.2013.11.020
- Primary Citation of Related Structures:
4HSC - PubMed Abstract:
Cholesterol-dependent cytolysins (CDCs) are a large family of bacterial toxins that exhibit a dependence on the presence of membrane cholesterol in forming large pores in cell membranes. Significant changes in the three-dimensional structure of these toxins are necessary to convert the soluble monomeric protein into a membrane pore. We have determined the crystal structure of the archetypical member of the CDC family, streptolysin O (SLO), a virulence factor from Streptococcus pyogenes. The overall fold is similar to previously reported CDC structures, although the C-terminal domain is in a different orientation with respect to the rest of the molecule. Surprisingly, a signature stretch of CDC sequence called the undecapeptide motif, a key region involved in membrane recognition, adopts a very different structure in SLO to that of the well-characterized CDC perfringolysin O (PFO), although the sequences in this region are identical. An analysis reveals that, in PFO, there are complementary interactions between the motif and the rest of domain 4 that are lost in SLO. Molecular dynamics simulations suggest that the loss of a salt bridge in SLO and a cation-pi interaction are determining factors in the extended conformation of the motif, which in turn appears to result in a greater flexibility of the neighboring L1 loop that houses a cholesterol-sensing motif. These differences may explain the differing abilities of SLO and PFO to efficiently penetrate target cell membranes in the first step of toxin insertion into the membrane.
Organizational Affiliation:
ACRF Rational Drug Discovery Centre, Biota Structural Biology Laboratory, St. Vincent's Institute of Medical Research, Fitzroy, Victoria 3065, Australia.