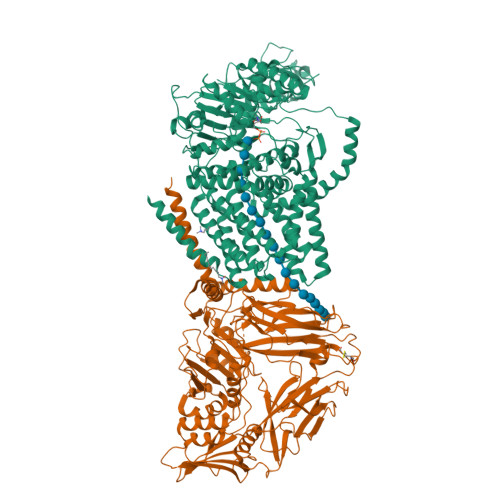
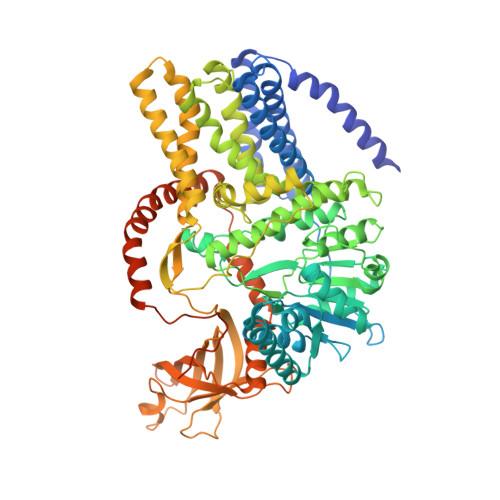
Crystallographic snapshot of cellulose synthesis and membrane translocation.
Morgan, J.L., Strumillo, J., Zimmer, J.(2012) Nature 493: 181-186
- PubMed: 23222542
- DOI: https://doi.org/10.1038/nature11744
- Primary Citation of Related Structures:
4HG6 - PubMed Abstract:
Cellulose, the most abundant biological macromolecule, is an extracellular, linear polymer of glucose molecules. It represents an essential component of plant cell walls but is also found in algae and bacteria. In bacteria, cellulose production frequently correlates with the formation of biofilms, a sessile, multicellular growth form. Cellulose synthesis and transport across the inner bacterial membrane is mediated by a complex of the membrane-integrated catalytic BcsA subunit and the membrane-anchored, periplasmic BcsB protein. Here we present the crystal structure of a complex of BcsA and BcsB from Rhodobacter sphaeroides containing a translocating polysaccharide. The structure of the BcsA-BcsB translocation intermediate reveals the architecture of the cellulose synthase, demonstrates how BcsA forms a cellulose-conducting channel, and suggests a model for the coupling of cellulose synthesis and translocation in which the nascent polysaccharide is extended by one glucose molecule at a time.
Organizational Affiliation:
Center for Membrane Biology, Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, Virginia 22908, USA.