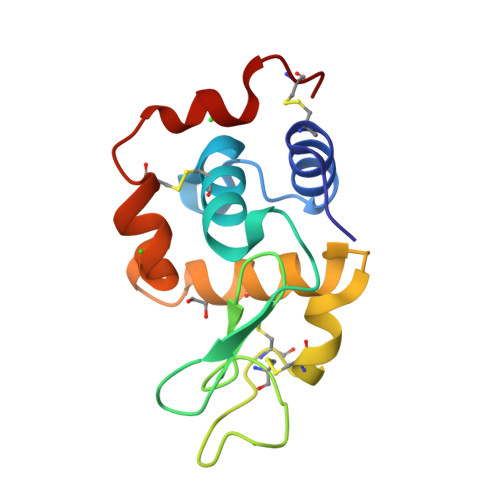
Insights into the mechanism of X-ray-induced disulfide-bond cleavage in lysozyme crystals based on EPR, optical absorption and X-ray diffraction studies.
Sutton, K.A., Black, P.J., Mercer, K.R., Garman, E.F., Owen, R.L., Snell, E.H., Bernhard, W.A.(2013) Acta Crystallogr D Biol Crystallogr 69: 2381-2394
- PubMed: 24311579
- DOI: https://doi.org/10.1107/S0907444913022117
- Primary Citation of Related Structures:
4H8X, 4H8Y, 4H8Z, 4H90, 4H91, 4H92, 4H93, 4H94, 4H9A, 4H9B, 4H9C, 4H9E, 4H9F, 4H9H, 4H9I - PubMed Abstract:
Electron paramagnetic resonance (EPR) and online UV-visible absorption microspectrophotometry with X-ray crystallography have been used in a complementary manner to follow X-ray-induced disulfide-bond cleavage. Online UV-visible spectroscopy showed that upon X-irradiation, disulfide radicalization appeared to saturate at an absorbed dose of approximately 0.5-0.8 MGy, in contrast to the saturating dose of ∼0.2 MGy observed using EPR at much lower dose rates. The observations suggest that a multi-track model involving product formation owing to the interaction of two separate tracks is a valid model for radiation damage in protein crystals. The saturation levels are remarkably consistent given the widely different experimental parameters and the range of total absorbed doses studied. The results indicate that even at the lowest doses used for structural investigations disulfide bonds are already radicalized. Multi-track considerations offer the first step in a comprehensive model of radiation damage that could potentially lead to a combined computational and experimental approach to identifying when damage is likely to be present, to quantitate it and to provide the ability to recover the native unperturbed structure.
Organizational Affiliation:
Hauptman-Woodward Medical Research Institute, 700 Ellicott Street, Buffalo, NY 14086, USA.