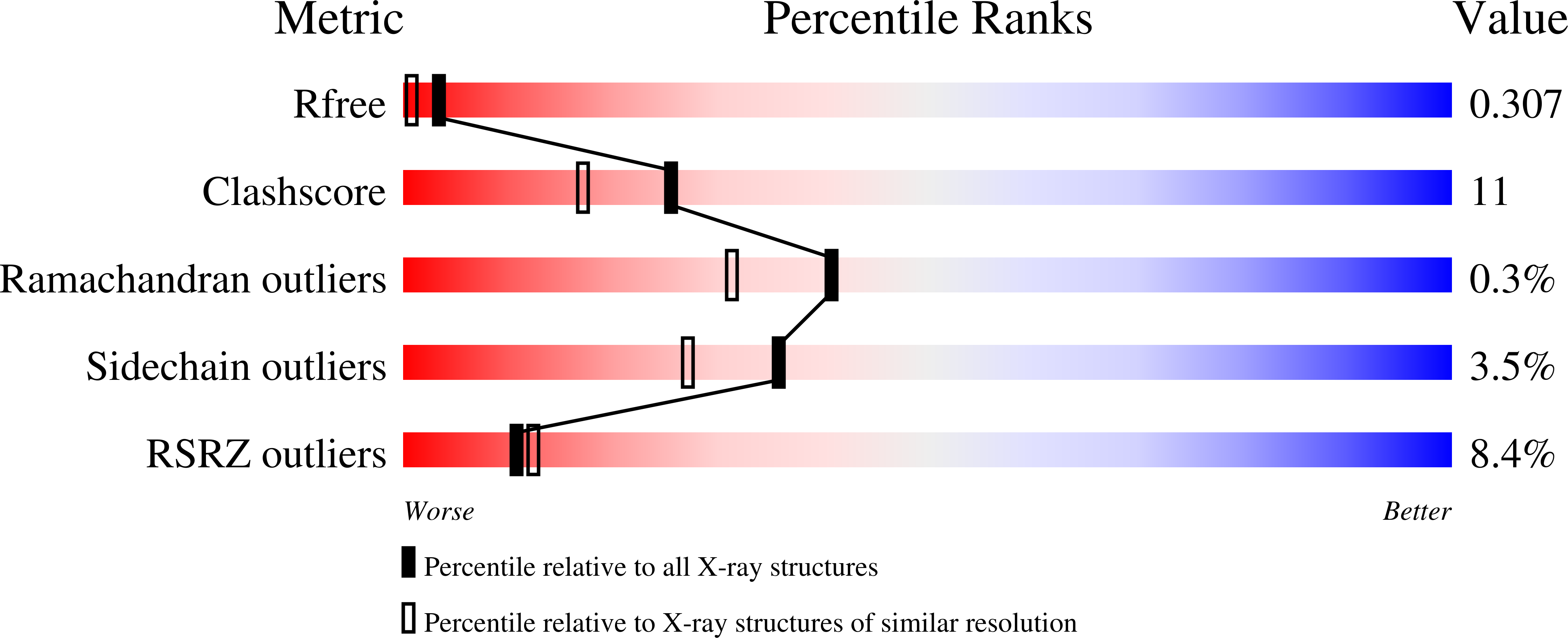
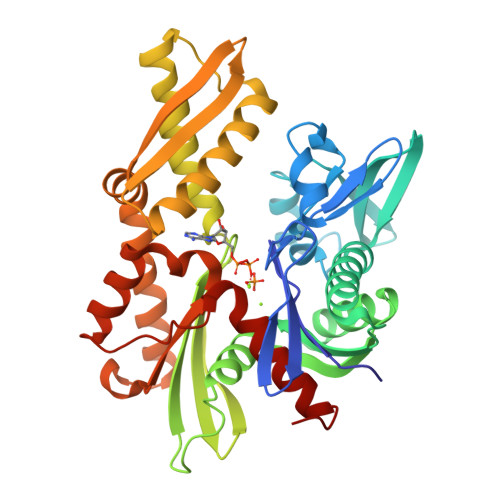
New crystal structures of HSC-70 ATP binding domain confirm the role of individual binding pockets and suggest a new method of inhibition.
Zhang, Z., Cellitti, J., Teriete, P., Pellecchia, M., Stec, B.(2015) Biochimie 108: 186-192
- PubMed: 25433210
- DOI: https://doi.org/10.1016/j.biochi.2014.11.012
- Primary Citation of Related Structures:
4H5N, 4H5R, 4H5T, 4H5V, 4H5W - PubMed Abstract:
In recent years the chaperone HSC-70 has become a target for drug design with a strong focus in anticancer therapies. In our study of possible inhibitors of HSC-70 enzymatic activity we screened compounds by NMR as well as X-ray crystallography. As part of our screening efforts we crystallized the human HSC-70 ATP binding domain and obtained novel crystal forms in addition to known structures. The new crystal structures highlight the mobility of the entire domain previously described by NMR, which was linked to its chaperone activity but not yet demonstrated by X-ray crystallography. Conformational changes across the entire molecule have been elucidated in response to the binding of small molecule ligands and show a pattern of mobility consistent with postulated signal transduction modes between the nucleotide binding domain (NBD) and the substrate binding domain (SBD). In addition, two crystal structures contained glycerol bound at a new site. Binding studies performed with glycerol analogs proved inhibitory properties of the site, which were further characterized by isothermal calorimetry and in silico docking studies. The presence of two binding pockets enabled us to explore a novel method of inhibition by compounds that bridge the adjacent phosphate and glycerol binding sites. Finally, an example of such a bridged inhibitor is proposed.
Organizational Affiliation:
Sanford-Burnham Medical Research Institute, 10901 N. Torrey Pines Rd., La Jolla, CA 92037, USA.