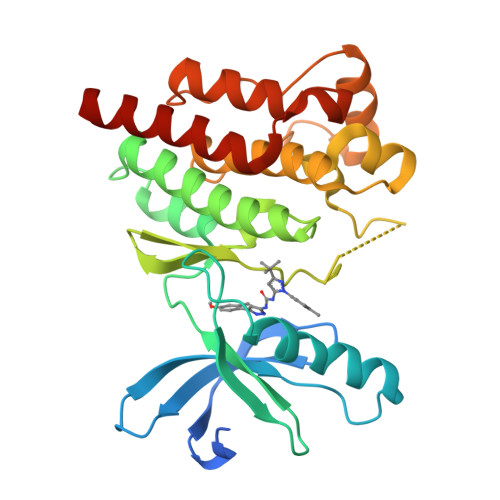
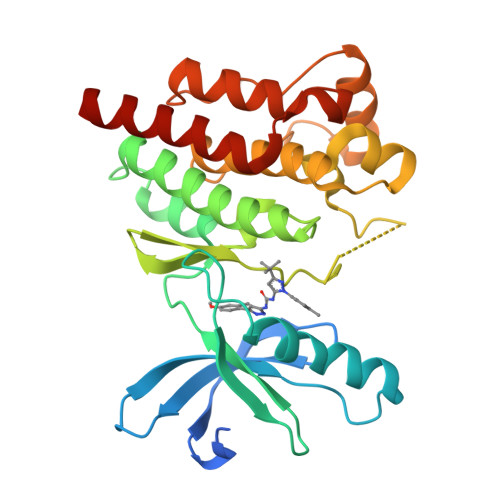
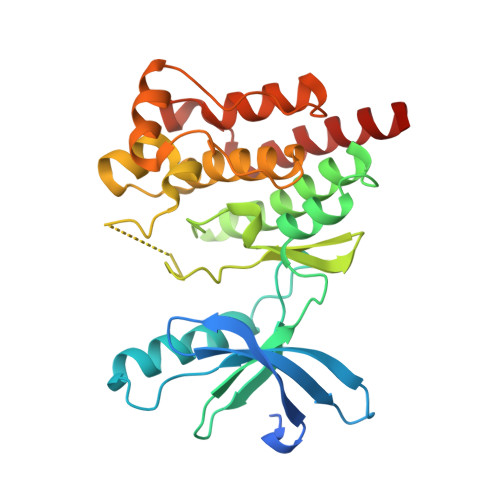
Identification of novel series of pyrazole and indole-urea based DFG-out PYK2 inhibitors.
Bhattacharya, S.K., Aspnes, G.E., Bagley, S.W., Boehm, M., Brosius, A.D., Buckbinder, L., Chang, J.S., Dibrino, J., Eng, H., Frederick, K.S., Griffith, D.A., Griffor, M.C., Guimaraes, C.R., Guzman-Perez, A., Han, S., Kalgutkar, A.S., Klug-McLeod, J., Garcia-Irizarry, C., Li, J., Lippa, B., Price, D.A., Southers, J.A., Walker, D.P., Wei, L., Xiao, J., Zawistoski, M.P., Zhao, X.(2012) Bioorg Med Chem Lett 22: 7523-7529
- PubMed: 23153798
- DOI: https://doi.org/10.1016/j.bmcl.2012.10.039
- Primary Citation of Related Structures:
4H1J, 4H1M - PubMed Abstract:
Previous drug discovery efforts identified classical PYK2 kinase inhibitors such as 2 and 3 that possess selectivity for PYK2 over its intra-family isoform FAK. Efforts to identify more kinome-selective chemical matter that stabilize a DFG-out conformation of the enzyme are described herein. Two sub-series of PYK2 inhibitors, an indole carboxamide-urea and a pyrazole-urea have been identified and found to have different binding interactions with the hinge region of PYK2. These leads proved to be more selective than the original classical inhibitors.
Organizational Affiliation:
Worldwide Medicinal Chemistry, Pfizer Global Research and Development, 620 Memorial Drive, Cambridge, MA 02139, United States. samit.k.bhattacharya@pfizer.com