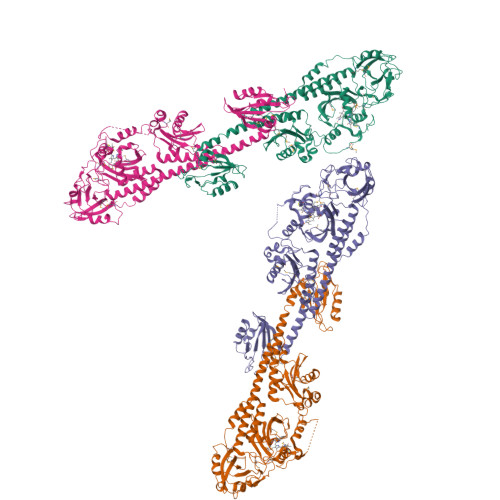
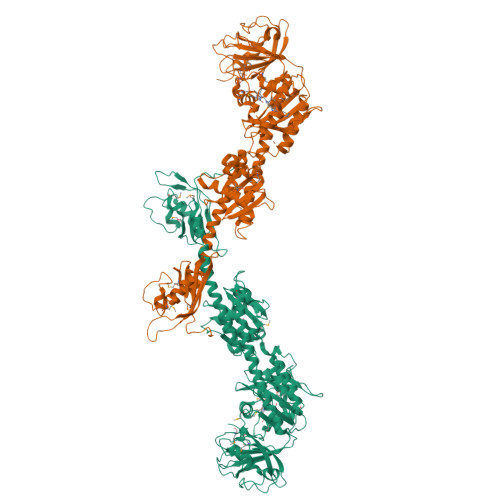
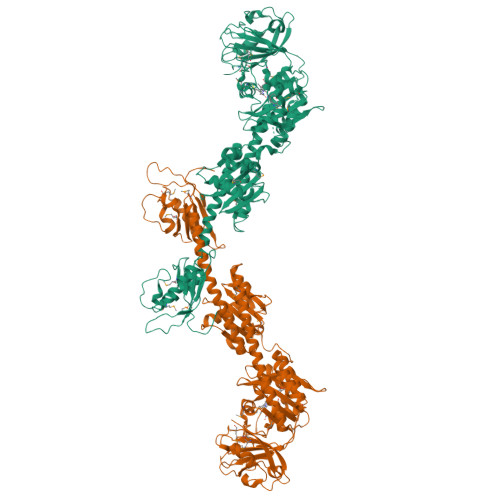
Structure of a bacteriophytochrome and light-stimulated protomer swapping with a gene repressor.
Bellini, D., Papiz, M.Z.(2012) Structure 20: 1436-1446
- PubMed: 22795083
- DOI: https://doi.org/10.1016/j.str.2012.06.002
- Primary Citation of Related Structures:
4GW9 - PubMed Abstract:
Phytochromes are photoreceptors in phototropic organisms that respond to light conditions by changing interactions between a response regulator and DNA. Bacterial phytochromes (BphPs) comprise an input photosensory core domain (PCD) and an output transducing domain (OTD). We report the structure of a BphP containing both PCD and the majority of its OTD, and demonstrate interaction with its cognate repressor. The OTD of RpBphP1, from Rhodopseudomonas palustris, is composed of a PAS/PAC domain and, to our knowledge, a hitherto unrecognized two-helix output sensor (HOS) domain. Unlike canonical BphPs, it does not transmit phosphorelay signals but forms a complex with the transcriptional repressor RpPpsR2 on photoconversion with far-red light. We show that HOS is essential for complex formation and that the anti-parallel dimer geometry is crucial in achieving HOS domain activation and protomer swapping under the control of light. These results provide insights into the steps taken by a two-component signaling system.
Organizational Affiliation:
Institute of Integrative Biology, Biosciences Building, University of Liverpool, Crown Street, Liverpool L69 7ZB, UK.