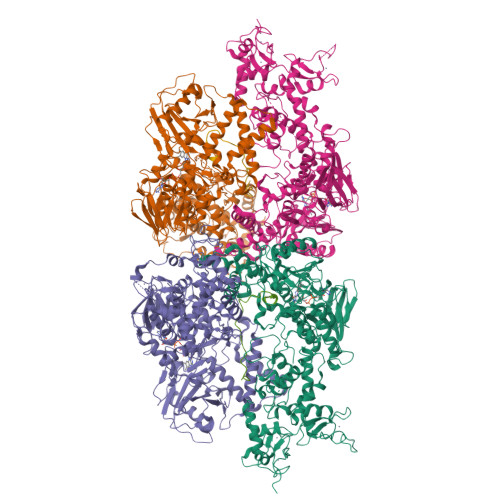
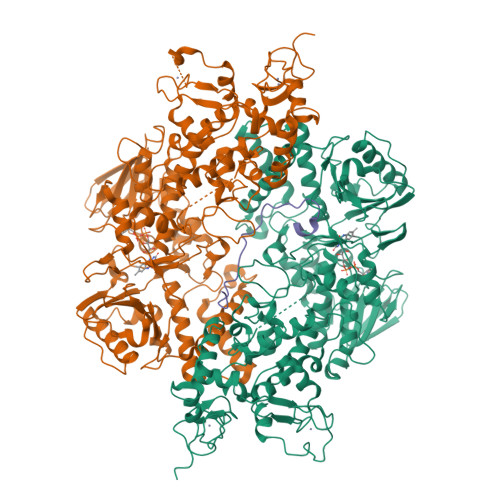
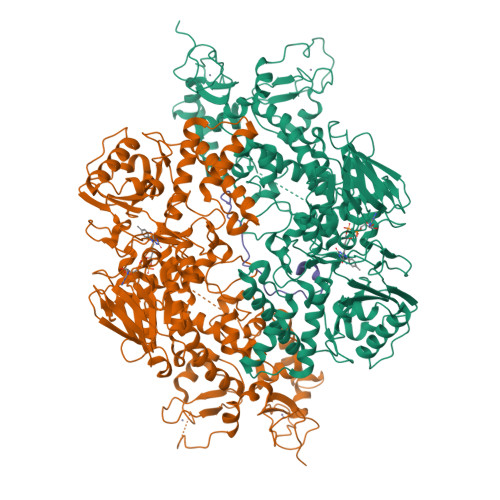
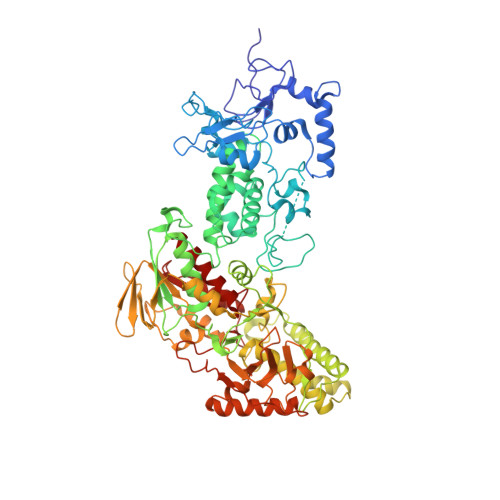
Structural insight into substrate recognition by histone demethylase LSD2/KDM1b
Chen, F., Yang, H., Dong, Z., Fang, J., Wang, P., Zhu, T., Gong, W., Fang, R., Shi, Y.G., Li, Z., Xu, Y.(2013) Cell Res 23: 306-309
- PubMed: 23357850
- DOI: https://doi.org/10.1038/cr.2013.17
- Primary Citation of Related Structures:
4GU0, 4HSU