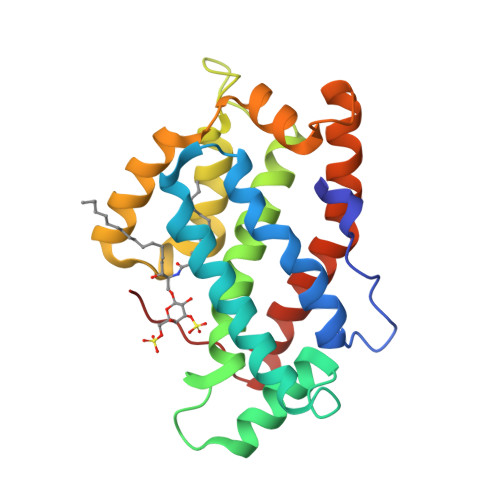
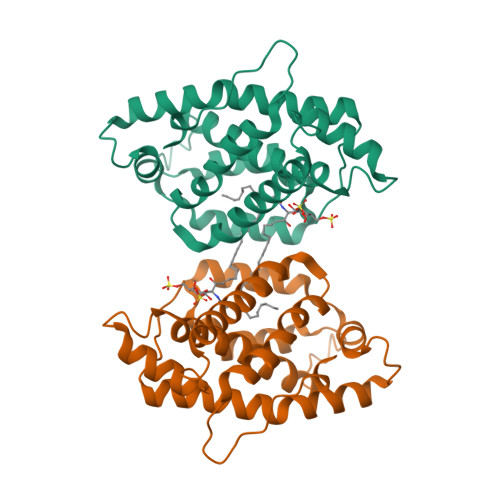
Structural insights into lipid-dependent reversible dimerization of human GLTP.
Samygina, V.R., Ochoa-Lizarralde, B., Popov, A.N., Cabo-Bilbao, A., Goni-de-Cerio, F., Molotkovsky, J.G., Patel, D.J., Brown, R.E., Malinina, L.(2013) Acta Crystallogr D Biol Crystallogr 69: 603-616
- PubMed: 23519669
- DOI: https://doi.org/10.1107/S0907444913000024
- Primary Citation of Related Structures:
4GH0, 4GHP, 4GHS, 4GIX, 4GJQ, 4GVT, 4GXD, 4GXG, 4H2Z - PubMed Abstract:
Human glycolipid transfer protein (hsGLTP) forms the prototypical GLTP fold and is characterized by a broad transfer selectivity for glycosphingolipids (GSLs). The GLTP mutation D48V near the `portal entrance' of the glycolipid binding site has recently been shown to enhance selectivity for sulfatides (SFs) containing a long acyl chain. Here, nine novel crystal structures of hsGLTP and the SF-selective mutant complexed with short-acyl-chain monoSF and diSF in different crystal forms are reported in order to elucidate the potential functional roles of lipid-mediated homodimerization. In all crystal forms, the hsGLTP-SF complexes displayed homodimeric structures supported by similarly organized intermolecular interactions. The dimerization interface always involved the lipid sphingosine chain, the protein C-terminus (C-end) and α-helices 6 and 2, but the D48V mutant displayed a `locked' dimer conformation compared with the hinge-like flexibility of wild-type dimers. Differences in contact angles, areas and residues at the dimer interfaces in the `flexible' and `locked' dimers revealed a potentially important role of the dimeric structure in the C-end conformation of hsGLTP and in the precise positioning of the key residue of the glycolipid recognition centre, His140. ΔY207 and ΔC-end deletion mutants, in which the C-end is shifted or truncated, showed an almost complete loss of transfer activity. The new structural insights suggest that ligand-dependent reversible dimerization plays a role in the function of human GLTP.
Organizational Affiliation:
Structural Biology Unit, CIC bioGUNE, Technology Park of Bizkaia, 48160 Derio, Spain.