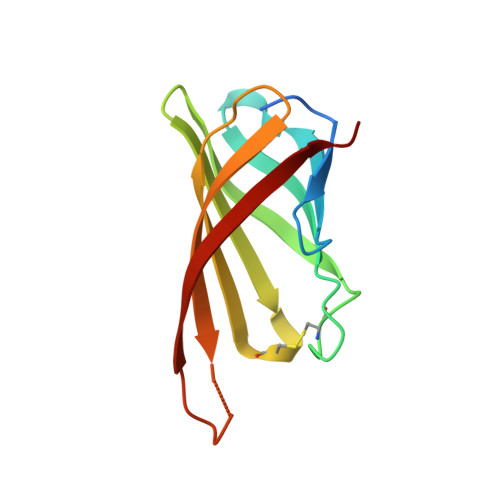
The highly dynamic oligomeric structure of bradavidin II is unique among avidin proteins.
Leppiniemi, J., Meir, A., Kahkonen, N., Kukkurainen, S., Maatta, J.A., Ojanen, M., Janis, J., Kulomaa, M.S., Livnah, O., Hytonen, V.P.(2013) Protein Sci 22: 980-994
- PubMed: 23661323
- DOI: https://doi.org/10.1002/pro.2281
- Primary Citation of Related Structures:
4GGR, 4GGT, 4GGZ - PubMed Abstract:
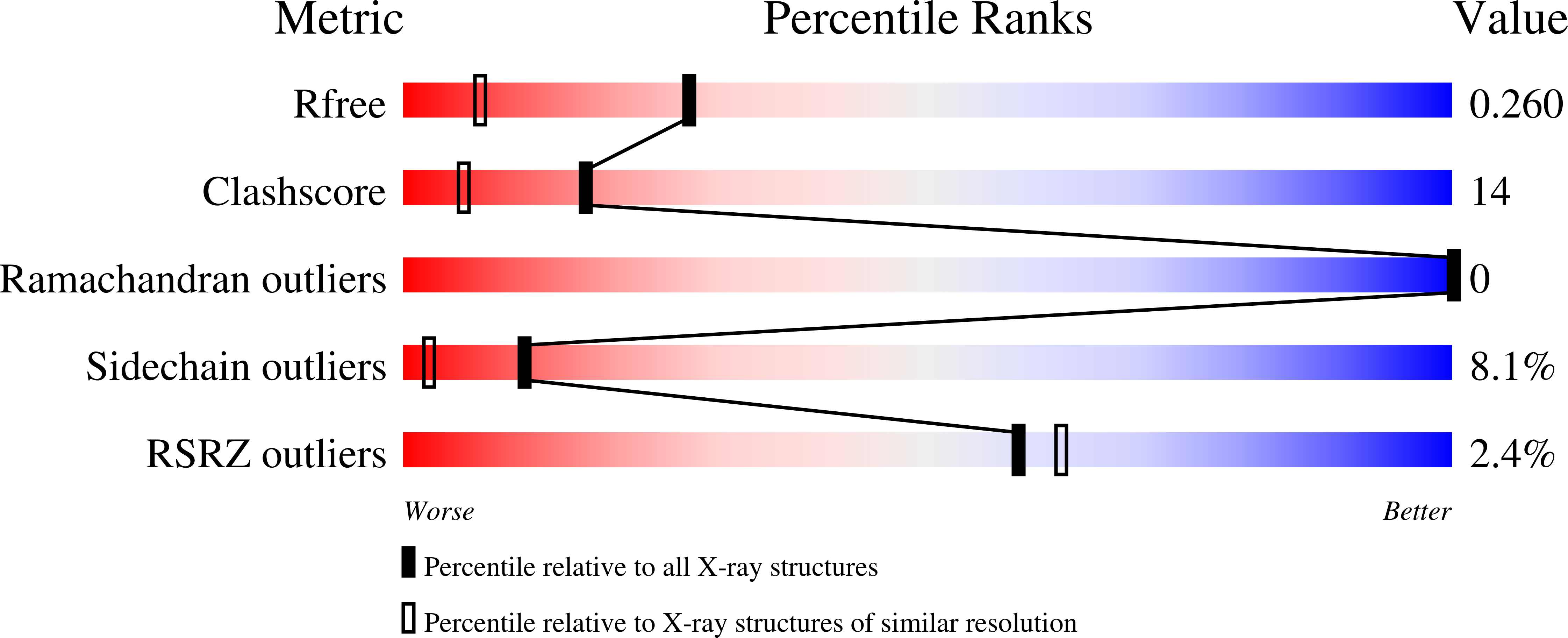
Bradavidin II is a biotin-binding protein from Bradyrhizobium japonicum that resembles chicken avidin and bacterial streptavidin. A biophysical characterization was carried out using dynamic light scattering, native mass spectrometry, differential scanning calorimetry, and isothermal titration calorimetry combined with structural characterization using X-ray crystallography. These observations revealed that bradavidin II differs from canonical homotetrameric avidin protein family members in its quaternary structure. In contrast with the other avidins, bradavidin II appears to have a dynamic (transient) oligomeric state in solution. It is monomeric at low protein concentrations but forms higher oligomeric assemblies at higher concentrations. The crystal structure of bradavidin II revealed an important role for Phe42 in shielding the bound ligand from surrounding water molecules, thus functionally replacing the L7,8 loop essential for tight ligand binding in avidin and streptavidin. This bradavidin II characterization opens new avenues for oligomerization-independent biotin-binding protein development.
Organizational Affiliation:
Institute of Biomedical Technology, University of Tampere and Tampere University Hospital, FI-33014, Tampere, Finland.