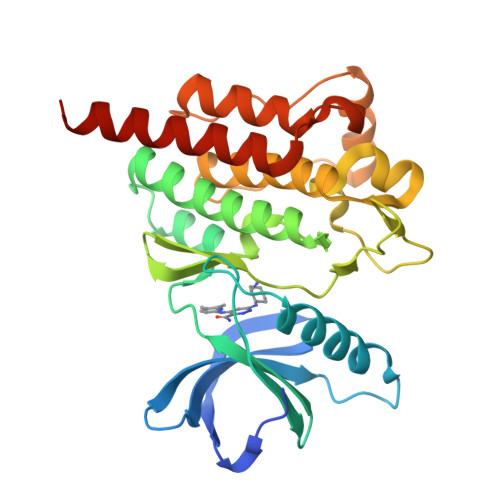
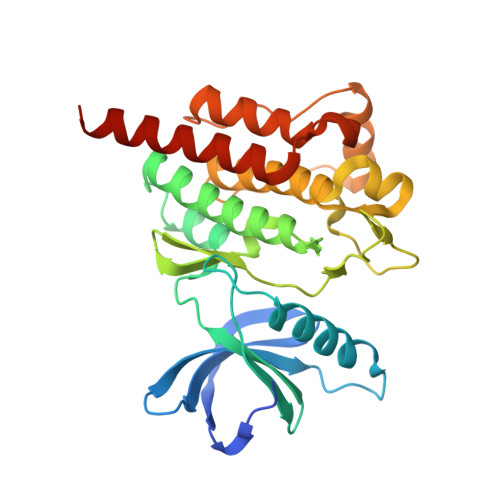
A specific SYK inhibitor blocks B Cell Receptor, Fc Receptor, and Toll-like Receptor 9 pathways for the treatment of inflammatory diseases.
Hsu, J., Kim, Y., Hu, D.-Q., Xu, D., Zhang, J., Pashine, A., Menke, J., Whittard, T., Romero, N., Truitt, T., Lukacs, C., Zhou, M., Lucas, M., Slade, M., DeMartino, J., Tan, S.-L., Liao, C.To be published.