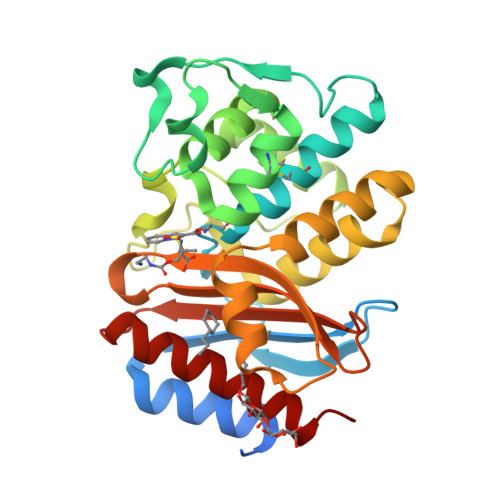
Structures of SHV-1 beta-lactamase with penem and penam sulfone inhibitors that form cyclic intermediates stabilized by carbonyl conjugation
Ke, W., Pattanaik, P., Bethel, C.R., Sheri, A., Buynak, J.D., Bonomo, R.A., van den Akker, F.(2012) PLoS One 7: e49035-e49035
- PubMed: 23145056
- DOI: https://doi.org/10.1371/journal.pone.0049035
- Primary Citation of Related Structures:
4GD6, 4GD8, 4GDB - PubMed Abstract:
Bacterial β-lactamase enzymes are in large part responsible for the decreased ability of β-lactam antibiotics to combat infections. The inability to overcome β-lactamase mediated resistance spurred the development of inhibitors with penems and penam sulfones being amongst the most potent and broad spectrum mechanism-based inactivators. These inhibitors form covalent, "suicide-type" inhibitory intermediates that are attached to the catalytic S70 residue. To further probe the details of the mechanism of β-lactamase inhibition by these novel compounds, we determined the crystal structures of SHV-1 bound with penem 1, and penam sulfones SA1-204 and SA3-53. Comparison with each other and with previously determined crystal structures of members of these classes of inhibitors suggests that the final conformation of the covalent adduct can vary greatly amongst the complex structures. In contrast, a common theme of carbonyl conjugation as a mechanism to avoid deacylation emerges despite that the penem and penam sulfone inhibitors form different types of intermediates. The detailed insights gained from this study could be used to further improve new mechanism-based inhibitors of these common class A serine β-lactamases.
Organizational Affiliation:
Department of Biochemistry, Case Western Reserve University, Cleveland, OH, USA.