Evolution of vitamin B(2) biosynthesis: eubacterial RibG and fungal Rib2 deaminases.
Chen, S.C., Shen, C.Y., Yen, T.M., Yu, H.C., Chang, T.H., Lai, W.L., Liaw, S.H.(2013) Acta Crystallogr D Biol Crystallogr 69: 227-236
- PubMed: 23385458
- DOI: https://doi.org/10.1107/S0907444912044903
- Primary Citation of Related Structures:
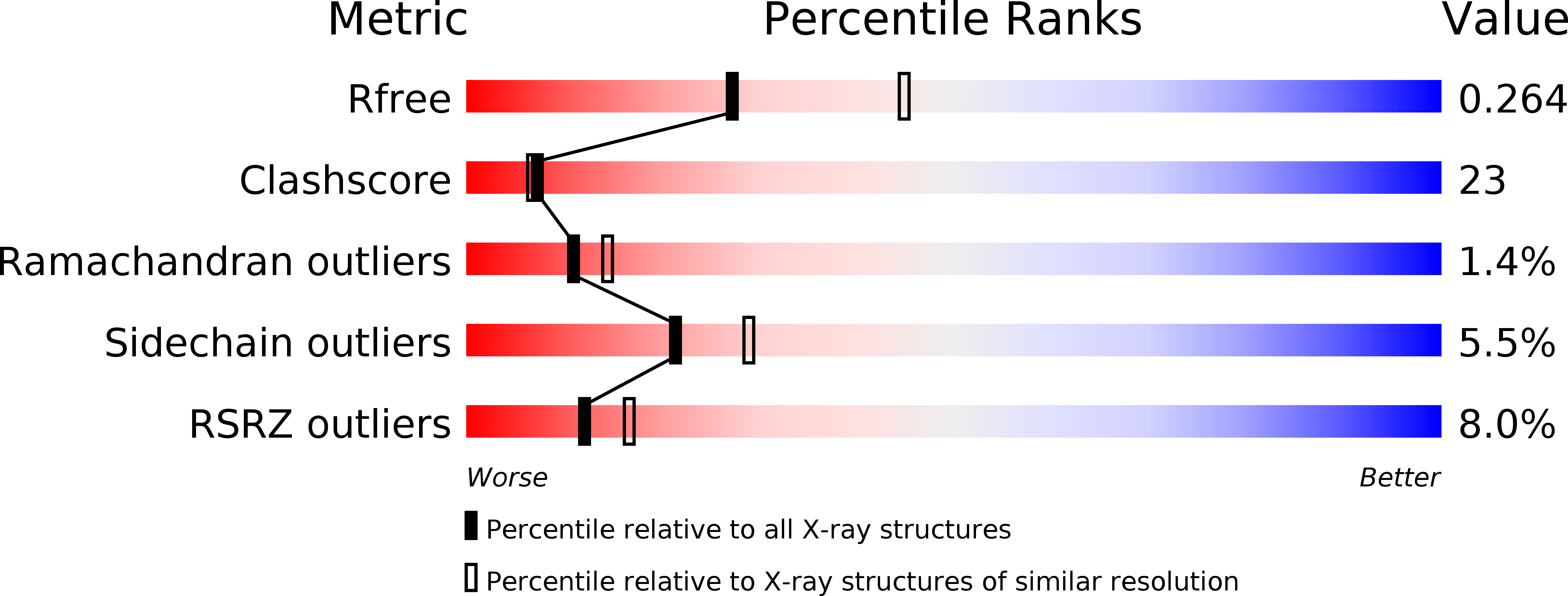
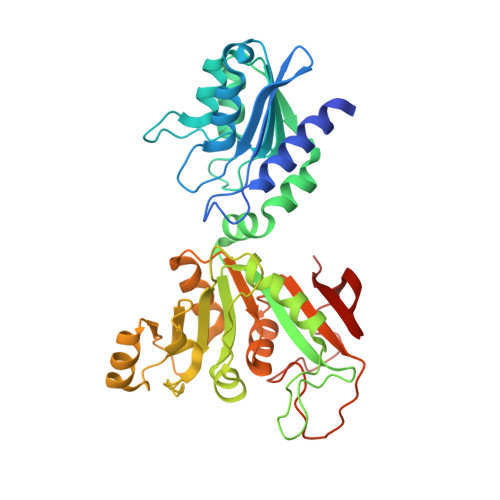
4G3M - PubMed Abstract:
Eubacterial RibG and yeast Rib2 possess a deaminase domain for pyrimidine deamination in the second and third steps, respectively, of riboflavin biosynthesis. These enzymes are specific for ribose and ribitol, respectively. Here, the crystal structure of Bacillus subtilis RibG in complex with a deaminase product is reported at 2.56 Å resolution. Two loops move towards the product on substrate binding, resulting in interactions with the ribosyl and phosphate groups and significant conformational changes. The product carbonyl moiety is bent out of the pyrimidine ring to coordinate to the catalytic zinc ion. Such distortions in the bound substrate and product may play an essential role in enzyme catalysis. The yeast Rib2 structure was modelled and a mutational analysis was carried out in order to understand the mechanism of substrate recognition in these two enzymes. Detailed structural comparisons revealed that the two consecutive carbonyl backbones that occur prior to the PCXXC signature constitute a binding hole for the target amino group of the substrate. This amino-binding hole is essential in B. subtilis RibG and is also conserved in the RNA/DNA-editing deaminases.
Organizational Affiliation:
Department of Life Sciences and Institute of Genome Sciences, National Yang-Ming University, Taipei 11221, Taiwan.