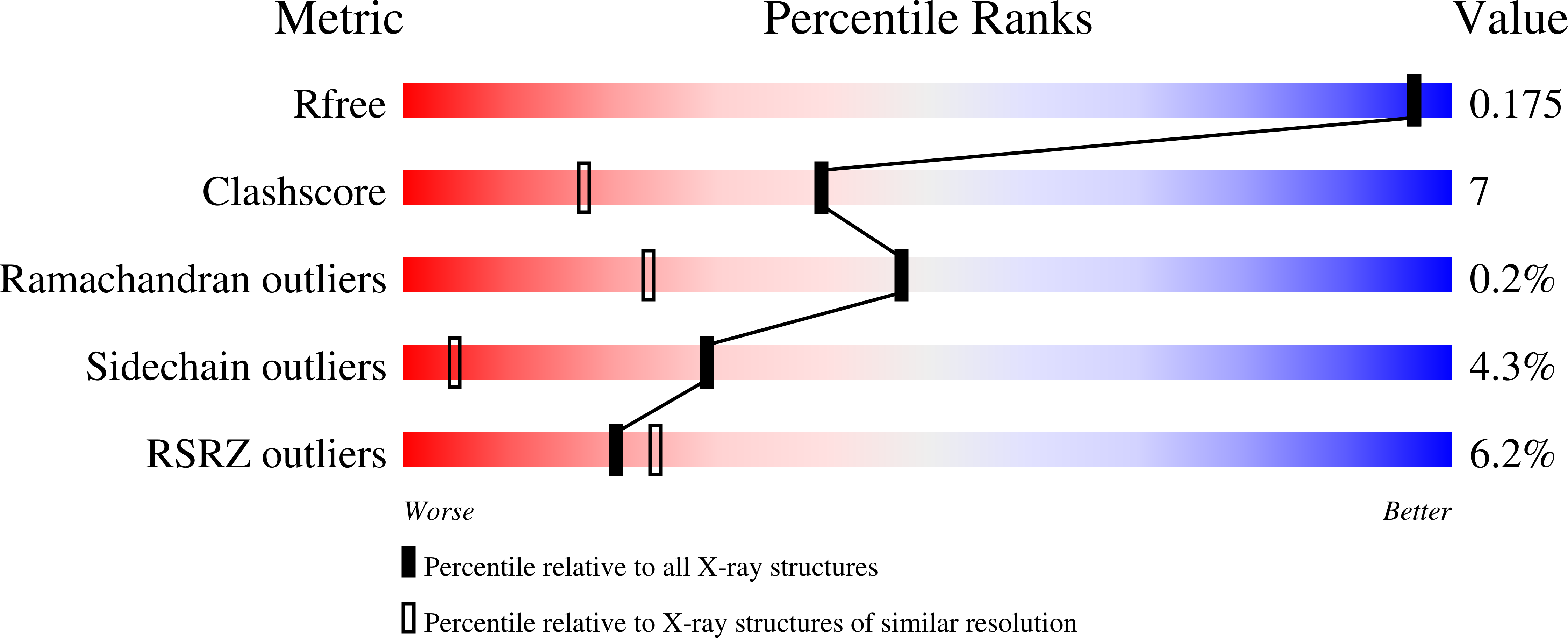
Mutational analysis of sulfite reductase hemoprotein reveals the mechanism for coordinated electron and proton transfer.
Smith, K.W., Stroupe, M.E.(2012) Biochemistry 51: 9857-9868
- PubMed: 23153334
- DOI: https://doi.org/10.1021/bi300947a
- Primary Citation of Related Structures:
4G38, 4G39, 4HTR - PubMed Abstract:
Sulfite reductase catalyzes the six-electron reduction of sulfite to sulfide. The active site, found in the hemoprotein subunit (SiRHP), sits on the distal face of a negatively charged porphyrinoid called siroheme whose central iron atom is coupled to a proximal Fe(4)S(4) cluster. Four positively charged amino acids are positioned around the active site cavity. Together, these two arginines (R83 and R153) and two lysines (K215 and K217) mitigate the negative charge on the siroheme macrocycle. They also serve as a cage around the distally bound anion that tightens when substrate binds and an active site loop clamps down. Structures of native SiRHP point to these amino acids as being important, but their specific roles are ill-defined. Here, we have altered those four active site amino acids and one amino acid on the flexible loop (N149) to probe their roles in SiRHP activity. None of these positively charged residues is required for electron transfer, but only R83S and N149W variants can produce a fully reduced product. By measuring the electrons used per unit of reduced sulfur released, we show that K215, R153, and K217 are responsible for intermediate and late proton transfers, whereas N149 and R153 play a role in the structure of the flexible loop that controls anion binding and release. R83 is primarily responsible for siroheme binding. Together, the activities and structures of these variants reveal specific roles for each in anion binding and in coupled proton transfer that facilitates electron transfer.
Organizational Affiliation:
Department of Biological Science and Institute of Molecular Biophysics, Florida State University, Tallahassee, FL 32306-4380, USA.