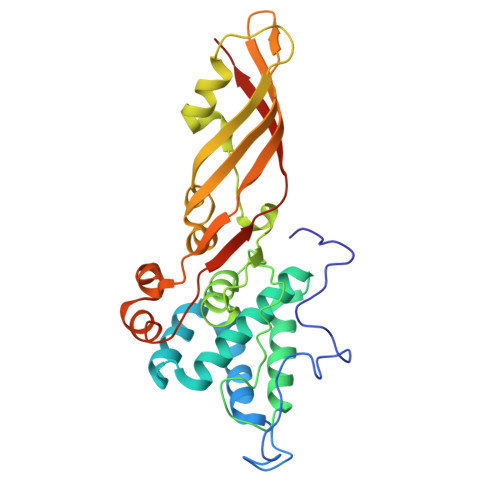
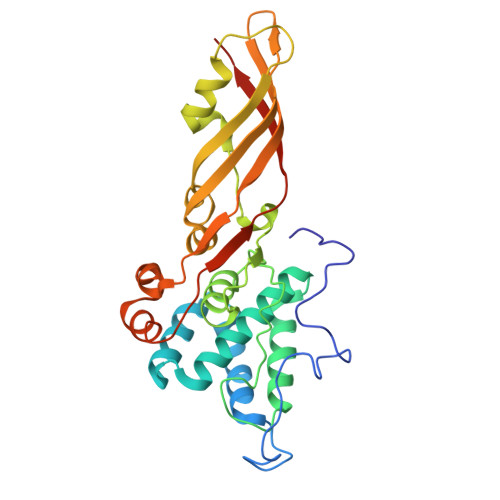
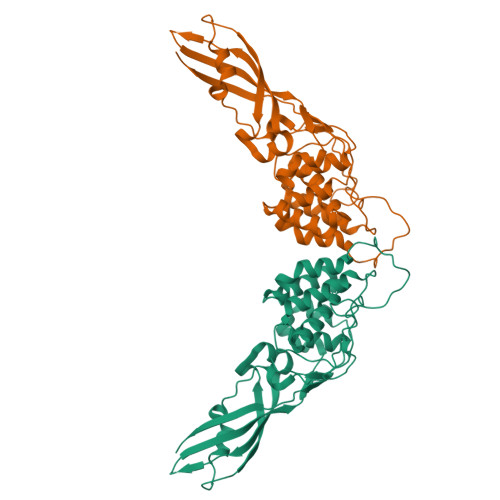
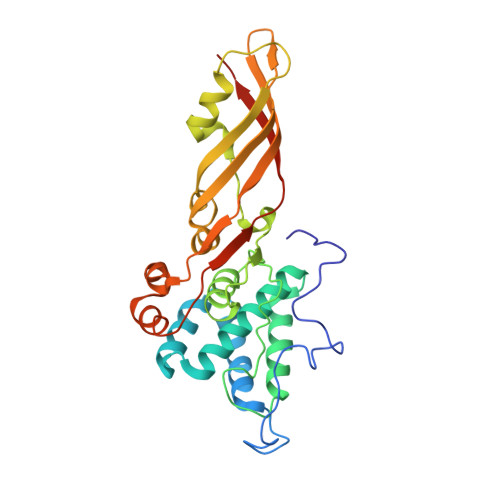
The Crystal Structure of the Lipid II-degrading Bacteriocin Syringacin M Suggests Unexpected Evolutionary Relationships between Colicin M-like Bacteriocins.
Grinter, R., Roszak, A.W., Cogdell, R.J., Milner, J.J., Walker, D.(2012) J Biological Chem 287: 38876-38888
- PubMed: 22995910
- DOI: https://doi.org/10.1074/jbc.M112.400150
- Primary Citation of Related Structures:
4FZL, 4FZM, 4FZN - PubMed Abstract:
Colicin-like bacteriocins show potential as next generation antibiotics with clinical and agricultural applications. Key to these potential applications is their high potency and species specificity that enables a single pathogenic species to be targeted with minimal disturbance of the wider microbial community. Here we present the structure and function of the colicin M-like bacteriocin, syringacin M from Pseudomonas syringae pv. tomato DC3000. Syringacin M kills susceptible cells through a highly specific phosphatase activity that targets lipid II, ultimately inhibiting peptidoglycan synthesis. Comparison of the structures of syringacin M and colicin M reveals that, in addition to the expected similarity between the homologous C-terminal catalytic domains, the receptor binding domains of these proteins, which share no discernible sequence homology, share a striking structural similarity. This indicates that the generation of the novel receptor binding and species specificities of these bacteriocins has been driven by diversifying selection rather than diversifying recombination as suggested previously. Additionally, the structure of syringacin M reveals the presence of an active site calcium ion that is coordinated by a conserved aspartic acid side chain and is essential for catalytic activity. We show that mutation of this residue to alanine inactivates syringacin M and that the metal ion is absent from the structure of the mutant protein. Consistent with the presence of Ca(2+) in the active site, we show that syringacin M activity is supported by Ca(2+), along with Mg(2+) and Mn(2+), and the protein is catalytically inactive in the absence of these ions.
Organizational Affiliation:
Institute of Infection, Immunity, and Inflammation, School of Life Sciences, College of Medical, Veterinary, and Life Sciences, University of Glasgow, Glasgow G12 8TA, Scotland, United Kingdom.