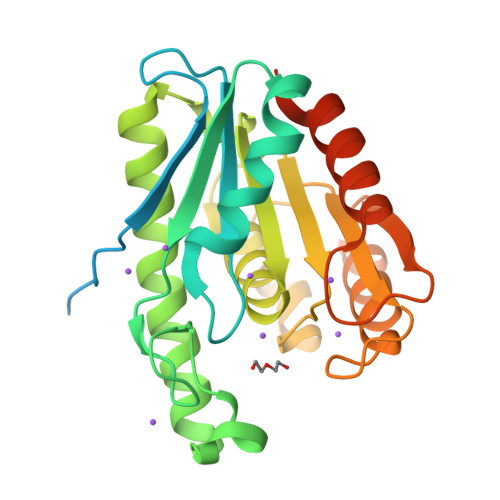
Enhanced enantioselectivity of a carboxyl esterase from Rhodobacter sphaeroides by directed evolution.
Ma, J., Wu, L., Guo, F., Gu, J., Tang, X., Jiang, L., Liu, J., Zhou, J., Yu, H.(2013) Appl Microbiol Biotechnol 97: 4897-4906
- PubMed: 22987200
- DOI: https://doi.org/10.1007/s00253-012-4396-2
- Primary Citation of Related Structures:
4FHZ, 4FTW - PubMed Abstract:
The present work created an esterase variant from Rhodobacter sphaeroides (RspE) with enhanced selectivity in hydrolytic kinetic resolutions by directed evolution. A "model" substrate, methyl mandelate, was introduced in the high-throughput screening procedure. E values of a variant CH (Asn62Cys/Leu145His) for six different esters were 10-83, which were a relative improvement compared to 2-20 for the wild type. Our subsequent crystal structure interpretation and molecular dynamics simulations helped shed light on the source of enantioselectivity modified by directed evolution. Though mutations displayed no "direct" interaction with the substrate, they were hypothesized to strengthen the intramolecular interaction in the catalytic cavity of variant. Conformation analysis revealed that the enhanced enantioselectivity of variant CH for the seven substrates applied in this study was derived from the decrease in size of the substrate binding pocket.
Organizational Affiliation:
Department of Chemical and Biological Engineering, Institute of Bioengineering, Zhejiang University, Hangzhou 310027, People's Republic of China.