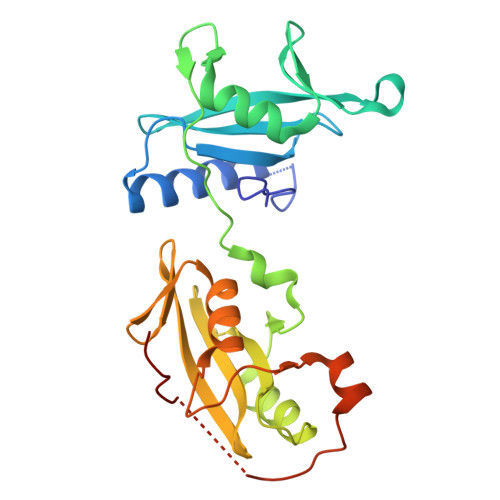
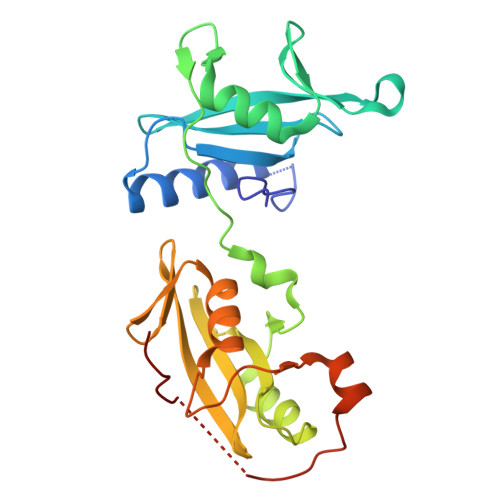
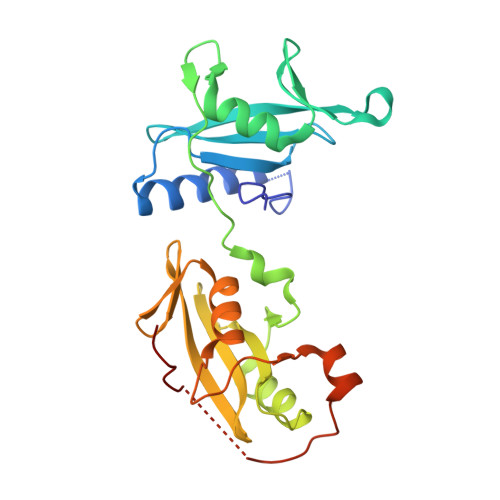
Structural and Functional Integration of the PLCgamma Interaction Domains Critical for Regulatory Mechanisms and Signaling Deregulation.
Bunney, T.D., Esposito, D., Mas-Droux, C., Lamber, E., Baxendale, R.W., Martins, M., Cole, A., Svergun, D., Driscoll, P.C., Katan, M.(2012) Structure 20: 2062-2075
- PubMed: 23063561
- DOI: https://doi.org/10.1016/j.str.2012.09.005
- Primary Citation of Related Structures:
4EY0, 4FBN - PubMed Abstract:
Multidomain proteins incorporating interaction domains are central to regulation of cellular processes. The elucidation of structural organization and mechanistic insights into many of these proteins, however, remain challenging due to their inherent flexibility. Here, we describe the organization and function of four interaction domains in PLCγ1 using a combination of structural biology and biochemical approaches. Intramolecular interactions within the regulatory region center on the cSH2 domain, the only domain that also interacts with the PLC-core. In the context of fibroblast growth-factor receptor signaling, the coordinated involvement of nSH2 and cSH2 domains mediates efficient phosphorylation of PLCγ1 resulting in the interruption of an autoinhibitory interface by direct competition and, independently, dissociation of PLCγ1 from the receptor. Further structural insights into the autoinhibitory surfaces provide a framework to interpret gain-of-function mutations in PLCγ isoforms linked to immune disorders and illustrate a distinct mechanism for regulation of PLC activity by common interaction domains.
Organizational Affiliation:
Institute of Structural and Molecular Biology, Division of Biosciences, University College London, Gower Street, London WC1E 6BT, UK.