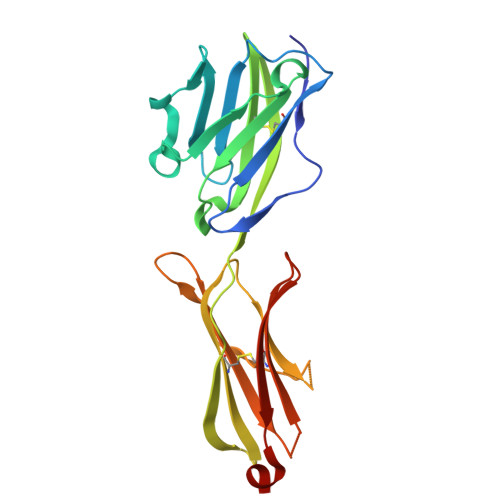
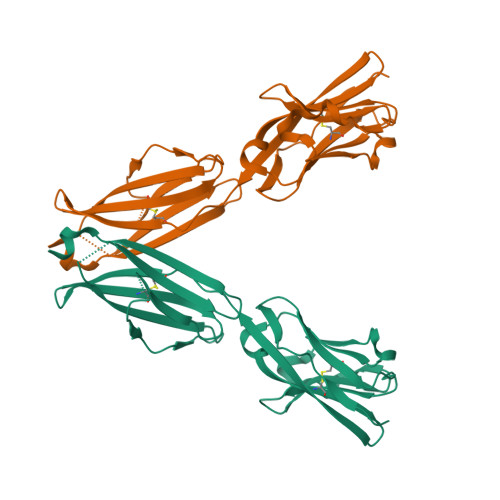
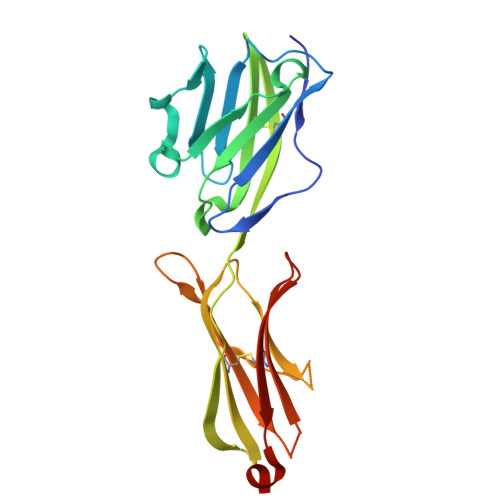
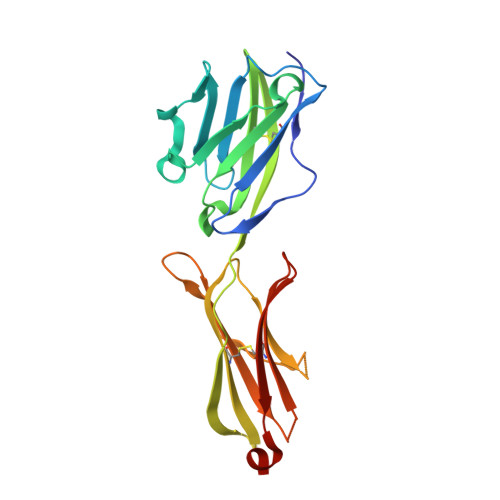
The molecular basis for modulation of human V(gamma)9V(delta)2 T cell responses by CD277/Butyrophilin-3 (BTN3A)-specific antibodies
Palakodeti, A., Sandstrom, A., Sundaresan, L., Harly, C., Nedellec, S., Olive, D., Scotet, E., Bonneville, M., Adams, E.J.(2012) J Biological Chem 287: 32780-32790
- PubMed: 22846996
- DOI: https://doi.org/10.1074/jbc.M112.384354
- Primary Citation of Related Structures:
4F80, 4F8Q, 4F8T, 4F9L, 4F9P - PubMed Abstract:
Human Vγ9Vδ2 T cells are well known for their rapid and potent response to infection and tumorigenesis when in the presence of endogenous or exogenous phosphoisoprenoids. However, the molecular mechanisms behind the activation of this γδ T cell population remains unclear. Evidence pointing to a role for the CD277/butyrophilin-3 (BTN3A) molecules in this response led us to investigate the structures of these molecules and their modifications upon binding to an agonist antibody (20.1) that mimics phosphoisoprenoid-mediated Vγ9Vδ2 activation and an antagonist antibody (103.2) that inhibits this reactivity. We find that the three BTN3A isoforms: BTN3A1, BTN3A2, and BTN3A3, have high structural homology to the B7 superfamily of proteins and exist as V-shaped homodimers in solution, associating through the membrane proximal C-type Ig domain. The 20.1 and 103.2 antibodies bind to separate epitopes on the BTN3A Ig-V domain with high affinity but likely with different valencies based on their binding orientation. These structures directly complement functional studies of this system that demonstrate that BTN3A1 is necessary for Vγ9Vδ2 activation and begin to unravel the extracellular events that occur during stimulation through the Vγ9Vδ2 T cell receptor.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Chicago, Chicago, Illinois 60637, USA.