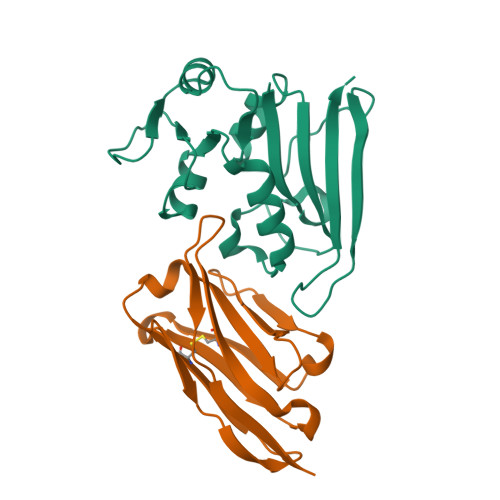
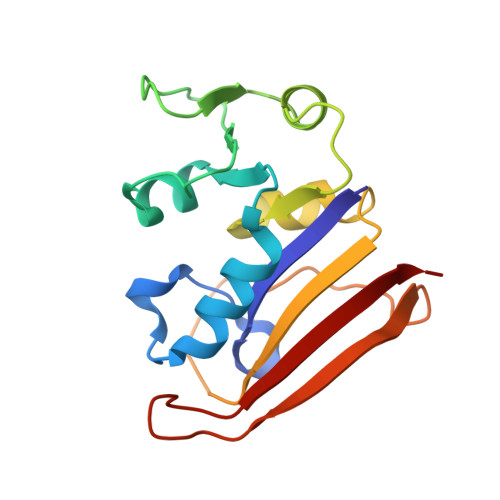
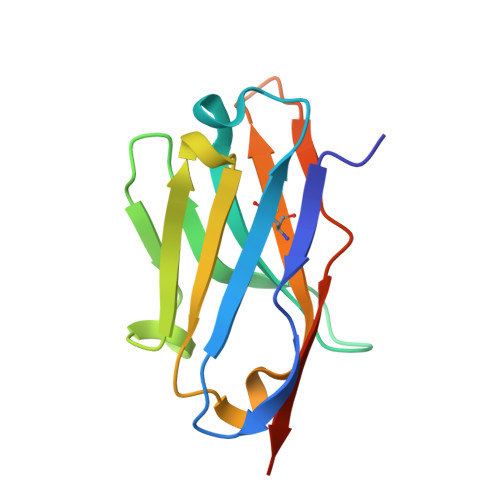
Mechanistic analysis of allosteric and non-allosteric effects arising from nanobody binding to two epitopes of the dihyrofolate reductase of Escherichia coli.
Oyen, D., Wechselberger, R., Srinivasan, V., Steyaert, J., Barlow, J.N.(2013) Biochim Biophys Acta 1834: 2147-2157
- PubMed: 23911607
- DOI: https://doi.org/10.1016/j.bbapap.2013.07.010
- Primary Citation of Related Structures:
4EIG, 4EIZ, 4EJ1, 4FHB - PubMed Abstract:
Although allosteric effector antibodies are used widely as modulators of receptors and enzymes, experimental analysis of their mechanism remains highly challenging. Here, we investigate the molecular mechanisms of allosteric and non-allosteric effector antibodies in an experimentally tractable system, consisting of single-domain antibodies (nanobodies) that target the model enzyme dihydrofolate reductase (DHFR) from Escherichia coli. A panel of thirty-five nanobodies was isolated using several strategies to increase nanobody diversity. The nanobodies exhibit a variety of effector properties, including partial inhibition, strong inhibition and stimulation of DHFR activity. Despite these diverse effector properties, chemical shift perturbation NMR epitope mapping identified only two epitope regions: epitope α is a new allosteric site that is over 10Å from the active site, while epitope β is located in the region of the Met20 loop. The structural basis for DHFR allosteric inhibition or activation upon nanobody binding to the α epitope was examined by solving the crystal structures of DHFR in complex with Nb113 (an allosteric inhibitor) and Nb179 (an allosteric activator). The structures suggest roles for conformational constraint and altered protein dynamics, but not epitope distortion, in the observed allosteric effects. The crystal structure of a β epitope region binder (ca1698) in complex with DHFR is also reported. Although CDR3 of ca1698 occupies the substrate binding site, ca1698 displays linear mixed inhibition kinetics instead of simple competitive inhibition kinetics. Two mechanisms are proposed to account for this apparent anomaly. Evidence for structural convergence of ca1698 and Nb216 during affinity maturation is also presented.
Organizational Affiliation:
Structural Biology Brussels, Vrije Universiteit Brussel, Pleinlaan 2, 1050 Brussels, Belgium; Structural Biology Research Centre, VIB, Pleinlaan 2, 1050 Brussels, Belgium.