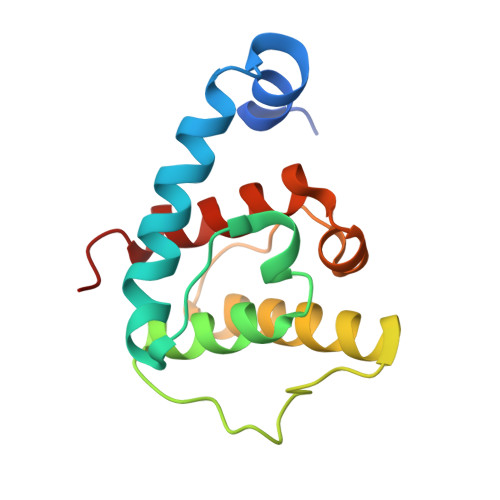
Structural basis for paxillin binding and focal adhesion targeting of beta-parvin.
Stiegler, A.L., Draheim, K.M., Li, X., Chayen, N.E., Calderwood, D.A., Boggon, T.J.(2012) J Biol Chem 287: 32566-32577
- PubMed: 22869380
- DOI: https://doi.org/10.1074/jbc.M112.367342
- Primary Citation of Related Structures:
4EDL, 4EDM, 4EDN - PubMed Abstract:
β-Parvin is a cytoplasmic adaptor protein that localizes to focal adhesions where it interacts with integrin-linked kinase and is involved in linking integrin receptors to the cytoskeleton. It has been reported that despite high sequence similarity to α-parvin, β-parvin does not bind paxillin, suggesting distinct interactions and cellular functions for these two closely related parvins. Here, we reveal that β-parvin binds directly and specifically to leucine-aspartic acid repeat (LD) motifs in paxillin via its C-terminal calponin homology (CH2) domain. We present the co-crystal structure of β-parvin CH2 domain in complex with paxillin LD1 motif to 2.9 Å resolution and find that the interaction is similar to that previously observed between α-parvin and paxillin LD1. We also present crystal structures of unbound β-parvin CH2 domain at 2.1 Å and 2.0 Å resolution that show significant conformational flexibility in the N-terminal α-helix, suggesting an induced fit upon paxillin binding. We find that β-parvin has specificity for the LD1, LD2, and LD4 motifs of paxillin, with K(D) values determined to 27, 42, and 73 μM, respectively, by surface plasmon resonance. Furthermore, we show that proper localization of β-parvin to focal adhesions requires both the paxillin and integrin-linked kinase binding sites and that paxillin is important for early targeting of β-parvin. These studies provide the first molecular details of β-parvin binding to paxillin and help define the requirements for β-parvin localization to focal adhesions.
Organizational Affiliation:
Department of Pharmacology, Yale University School of Medicine, New Haven, Connecticut 06520, USA.