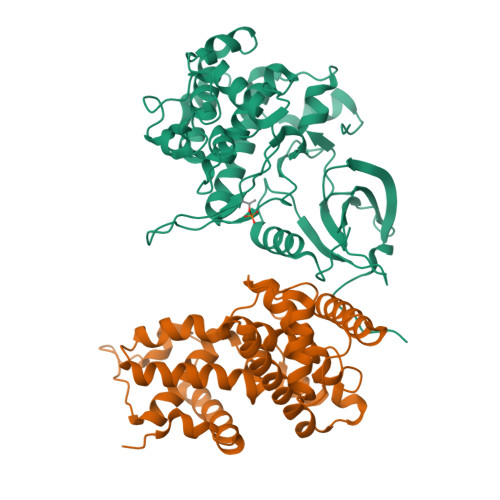
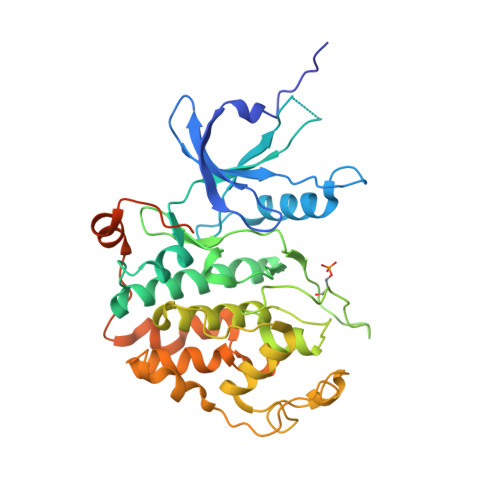
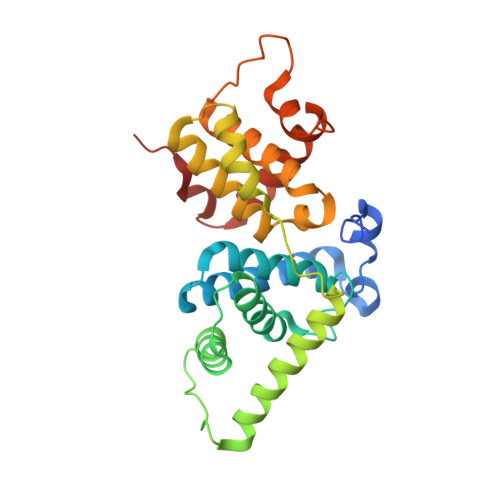
The CDK9 tail determines the reaction pathway of positive transcription elongation factor b.
Baumli, S., Hole, A.J., Wang, L.Z., Noble, M.E., Endicott, J.A.(2012) Structure 20: 1788-1795
- PubMed: 22959624
- DOI: https://doi.org/10.1016/j.str.2012.08.011
- Primary Citation of Related Structures:
4EC8, 4EC9 - PubMed Abstract:
CDK9, the kinase of positive transcription elongation factor b (P-TEFb), stimulates transcription elongation by phosphorylating RNA polymerase II and transcription elongation factors. Using kinetic analysis of a human P-TEFb complex consisting of CDK9 and cyclin T, we show that the CDK9 C-terminal tail sequence is important for the catalytic mechanism and imposes an ordered binding of substrates and release of products. Crystallographic analysis of a CDK9/cyclin T complex in which the C-terminal tail partially blocks the ATP binding site reveals a possible reaction intermediate. Biochemical characterization of CDK9 mutants supports a model in which the CDK9 tail cycles through different conformational states. We propose that this mechanism is critical for the pattern of CTD Ser2 phosphorylation on actively transcribed genes.
Organizational Affiliation:
Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne NE2 4HH, UK. sbaumli@gmx.ch