Chitin-induced dimerization activates a plant immune receptor.
Liu, T., Liu, Z., Song, C., Hu, Y., Han, Z., She, J., Fan, F., Wang, J., Jin, C., Chang, J., Zhou, J.M., Chai, J.(2012) Science 336: 1160-1164
- PubMed: 22654057
- DOI: https://doi.org/10.1126/science.1218867
- Primary Citation of Related Structures:
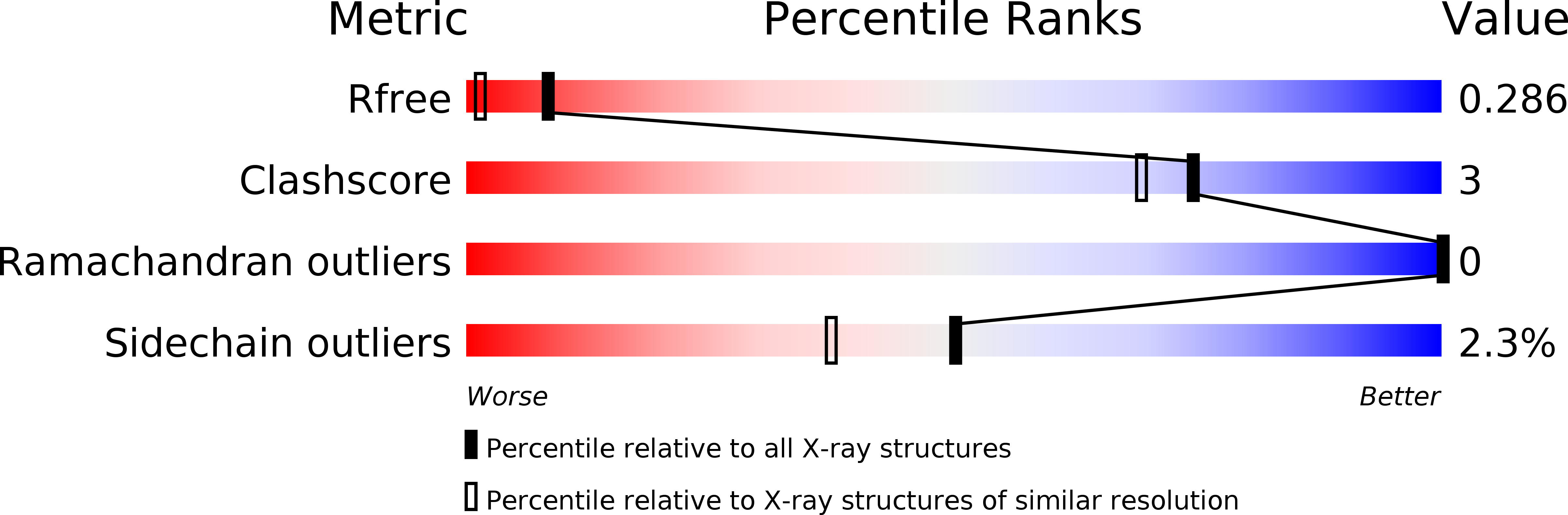
4EBY, 4EBZ - PubMed Abstract:
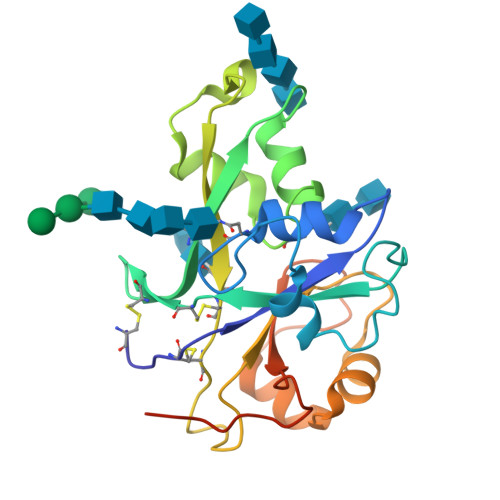
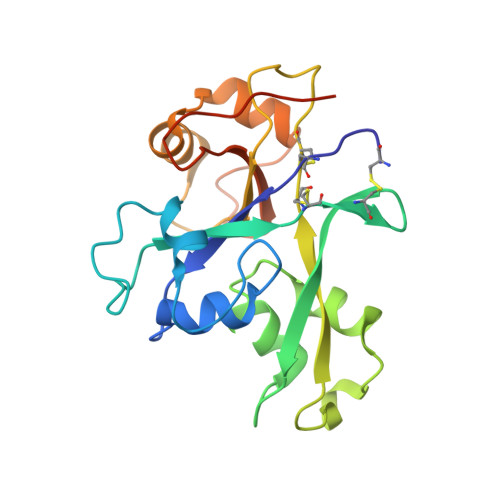
Pattern recognition receptors confer plant resistance to pathogen infection by recognizing the conserved pathogen-associated molecular patterns. The cell surface receptor chitin elicitor receptor kinase 1 of Arabidopsis (AtCERK1) directly binds chitin through its lysine motif (LysM)-containing ectodomain (AtCERK1-ECD) to activate immune responses. The crystal structure that we solved of an AtCERK1-ECD complexed with a chitin pentamer reveals that their interaction is primarily mediated by a LysM and three chitin residues. By acting as a bivalent ligand, a chitin octamer induces AtCERK1-ECD dimerization that is inhibited by shorter chitin oligomers. A mutation attenuating chitin-induced AtCERK1-ECD dimerization or formation of nonproductive AtCERK1 dimer by overexpression of AtCERK1-ECD compromises AtCERK1-mediated signaling in plant cells. Together, our data support the notion that chitin-induced AtCERK1 dimerization is critical for its activation.
Organizational Affiliation:
Graduate Program in Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.