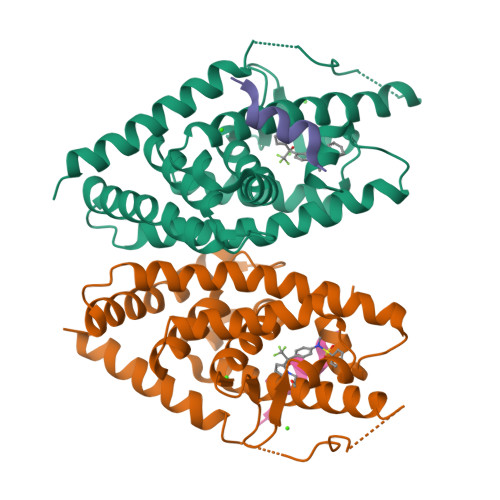
Discovery of a new binding mode for a series of liver X receptor agonists.
Kopecky, D.J., Jiao, X.Y., Fisher, B., McKendry, S., Labelle, M., Piper, D.E., Coward, P., Shiau, A.K., Escaron, P., Danao, J., Chai, A., Jaen, J., Kayser, F.(2012) Bioorg Med Chem Lett 22: 2407-2410
- PubMed: 22406115
- DOI: https://doi.org/10.1016/j.bmcl.2012.02.028
- Primary Citation of Related Structures:
4DK7, 4DK8 - PubMed Abstract:
Structural modification of a series of dual LXRα/β agonists led to the identification of a new class of LXRβ partial agonists. An X-ray co-crystal structure shows that a representative member of this series, pyrrole 5, binds to LXRβ with a reversed orientation compared to 1.
Organizational Affiliation:
Department of Medicinal Chemistry, Amgen Inc., 1120 Veterans Blvd., South San Francisco, CA 94080, USA. dkopecky@amgen.com