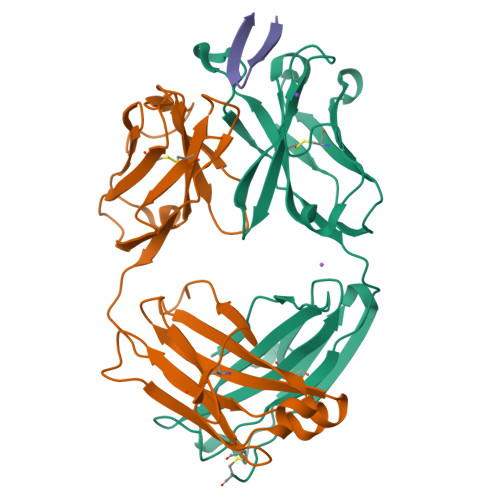
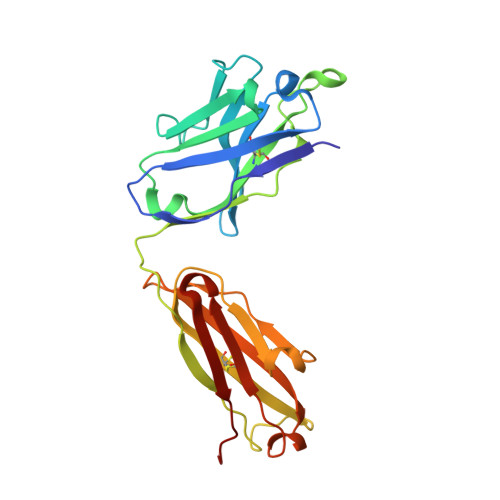
Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1.
Kong, L., Giang, E., Robbins, J.B., Stanfield, R.L., Burton, D.R., Wilson, I.A., Law, M.(2012) Proc Natl Acad Sci U S A 109: 9499-9504
- PubMed: 22623528
- DOI: https://doi.org/10.1073/pnas.1202924109
- Primary Citation of Related Structures:
4DGV, 4DGY - PubMed Abstract:
Hepatitis C virus (HCV) infects more than 2% of the global population and is a leading cause of liver cirrhosis, hepatocellular carcinoma, and end-stage liver diseases. Circulating HCV is genetically diverse, and therefore a broadly effective vaccine must target conserved T- and B-cell epitopes of the virus. Human mAb HCV1 has broad neutralizing activity against HCV isolates from at least four major genotypes and protects in the chimpanzee model from primary HCV challenge. The antibody targets a conserved antigenic site (residues 412-423) on the virus E2 envelope glycoprotein. Two crystal structures of HCV1 Fab in complex with an epitope peptide at 1.8-Å resolution reveal that the epitope is a β-hairpin displaying a hydrophilic face and a hydrophobic face on opposing sides of the hairpin. The antibody predominantly interacts with E2 residues Leu(413) and Trp(420) on the hydrophobic face of the epitope, thus providing an explanation for how HCV isolates bearing mutations at Asn(415) on the same binding face escape neutralization by this antibody. The results provide structural information for a neutralizing epitope on the HCV E2 glycoprotein and should help guide rational design of HCV immunogens to elicit similar broadly neutralizing antibodies through vaccination.
Organizational Affiliation:
Departments of Molecular Biology, International AIDS Vaccine Initiative Neutralizing Antibody Center, and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.