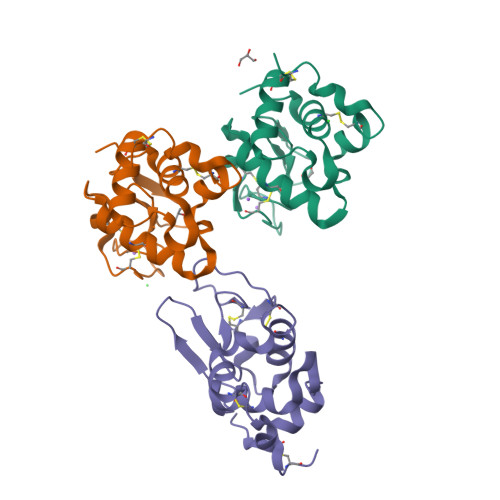
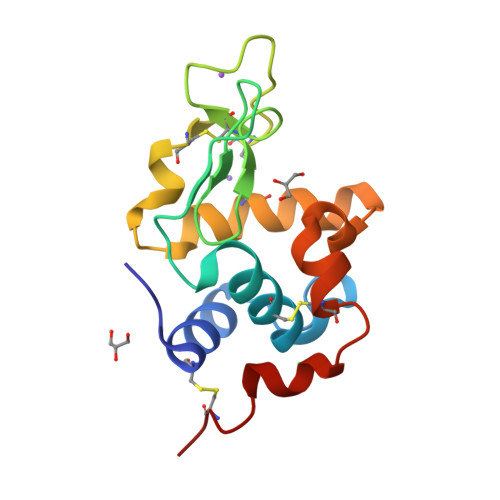
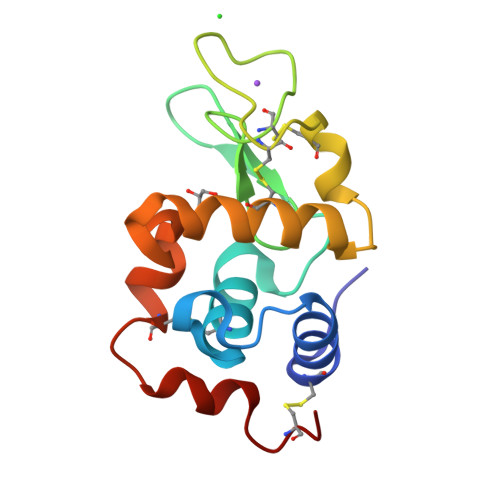
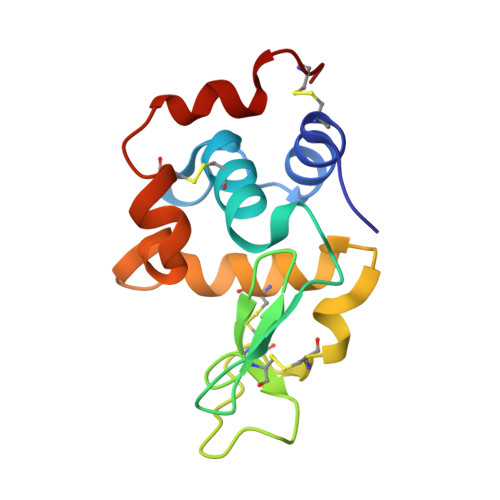
Characterization of heat induced spherulites of lysozyme reveals new insight on amyloid initiation
Sharma, P., Verma, N., Singh, P.K., Korpole, S., Ashish(2016) Sci Rep 6: 22475-22475
- PubMed: 26926993
- DOI: https://doi.org/10.1038/srep22475
- Primary Citation of Related Structures:
4D9Z, 4DC4, 4EOF, 4II8, 4R0F - PubMed Abstract:
Here, we report results obtained during our experiments to visualize how heat transforms globular protein, lysozyme into building block of β-amyloids. Light scattering experiments showed formation of lower order associated species around 50-70 °C followed by rapid cooperativity to β-amyloid fibrils. Interestingly, crystallization drops set at higher temperatures either led to aggregates or spherulites. The latter possess an amorphous β-fibril rich core with thin crystalline needles projecting outwards. Diffraction of the crystalline outgrowths revealed novel dimers and trimers of lysozyme where individual chains were similar to monomer with marginal gain in β-sheet content. Importantly, analysis of Amide I stretching frequencies showed that protein loses its secondary structure at temperatures higher than where we obtained crystals followed by rapid gain in β-sheet content. Interestingly, attempts to use the needles as seeds for more crystals led to "broom-like" fibril formations at the ends. Further, aggregation inhibitors like arginine and benzyl alcohol completely obliterated spherulites formation during crystallization. Refinement of crystals of lysozyme in presence of these molecules showed these small molecules bind to the interfaces of heat associated dimers and trimers. Overall our work concludes that heat induced weakly associated structures of lysozyme are the first step towards its amyloid formation.
Organizational Affiliation:
CSIR-Institute of Microbial Technology, Chandigarh, India.