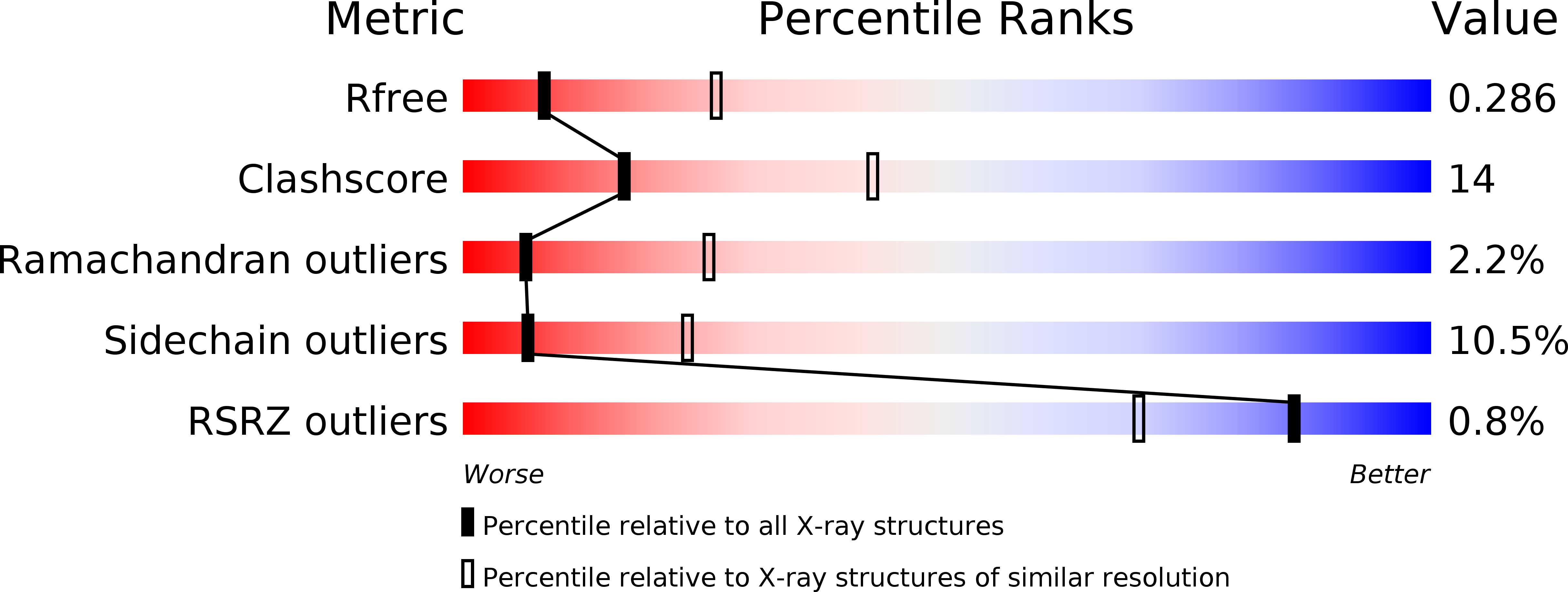
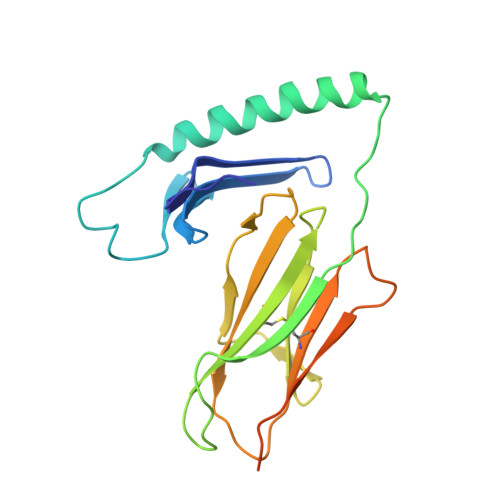
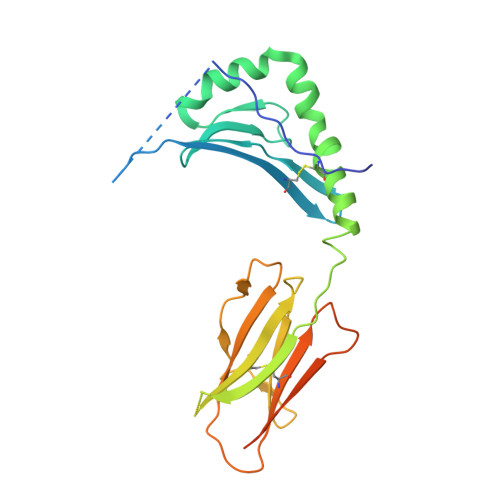
Structural and functional studies of trans-encoded HLA-DQ2.3 (DQA1*03:01/DQB1*02:01) protein molecule
Tollefsen, S., Hotta, K., Chen, X., Simonsen, B., Swaminathan, K., Mathews, I.I., Sollid, L.M., Kim, C.-Y.(2012) J Biol Chem 287: 13611-13619
- PubMed: 22362761
- DOI: https://doi.org/10.1074/jbc.M111.320374
- Primary Citation of Related Structures:
4D8P - PubMed Abstract:
MHC class II molecules are composed of one α-chain and one β-chain whose membrane distal interface forms the peptide binding groove. Most of the existing knowledge on MHC class II molecules comes from the cis-encoded variants where the α- and β-chain are encoded on the same chromosome. However, trans-encoded class II MHC molecules, where the α- and β-chain are encoded on opposite chromosomes, can also be expressed. We have studied the trans-encoded class II HLA molecule DQ2.3 (DQA1*03:01/DQB1*02:01) that has received particular attention as it may explain the increased risk of certain individuals to type 1 diabetes. We report the x-ray crystal structure of this HLA molecule complexed with a gluten epitope at 3.05 Å resolution. The gluten epitope, which is the only known HLA-DQ2.3-restricted epitope, is preferentially recognized in the context of the DQ2.3 molecule by T-cell clones of a DQ8/DQ2.5 heterozygous celiac disease patient. This preferential recognition can be explained by improved HLA binding as the epitope combines the peptide-binding motif of DQ2.5 (negative charge at P4) and DQ8 (negative charge at P1). The analysis of the structure of DQ2.3 together with all other available DQ crystal structures and sequences led us to categorize DQA1 and DQB1 genes into two groups where any α-chain and β-chain belonging to the same group are expected to form a stable heterodimer.
Organizational Affiliation:
Centre for Immune Regulation and Department of Immunology, University of Oslo and Oslo University Hospital, Rikshospitalet, 0027 Oslo, Norway.