A mechanistic basis for converting a receptor tyrosine kinase agonist to an antagonist.
Kang, Y.J., Kim, K.L., Cho, H.S., Chung, J.H.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HEPATOCYTE GROWTH FACTOR | 193 | Homo sapiens | Mutation(s): 1 | 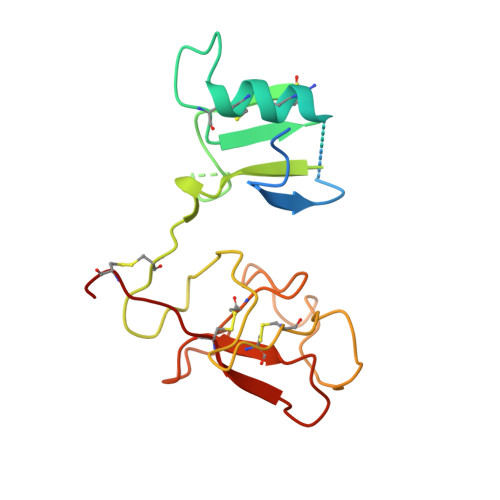 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P14210 (Homo sapiens) Explore P14210 Go to UniProtKB: P14210 | |||||
PHAROS: P14210 GTEx: ENSG00000019991 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P14210 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SFN68 FAB | B [auth H] | 218 | Oryctolagus cuniculus | Mutation(s): 0 | 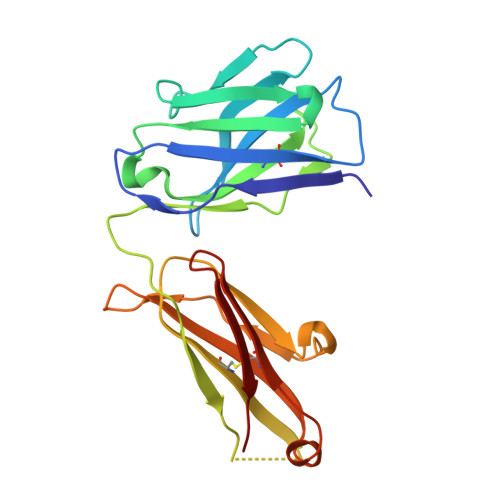 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SFN68 FAB | C [auth L] | 215 | Oryctolagus cuniculus | Mutation(s): 0 | 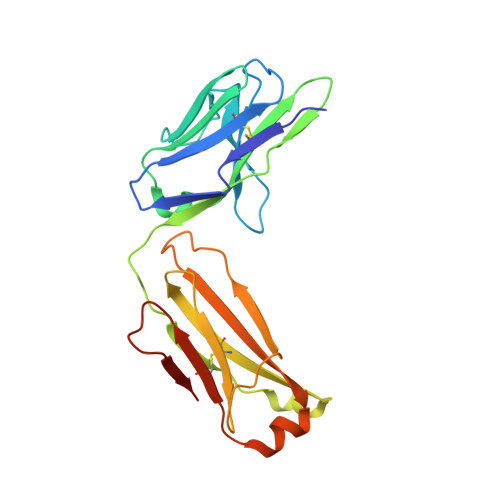 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01834 (Homo sapiens) Explore P01834 Go to UniProtKB: P01834 | |||||
PHAROS: P01834 | |||||
Find proteins for U3KM01 (Oryctolagus cuniculus) Explore U3KM01 Go to UniProtKB: U3KM01 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | U3KM01P01834 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 86.703 | α = 90 |
| b = 297.692 | β = 90 |
| c = 70.713 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |