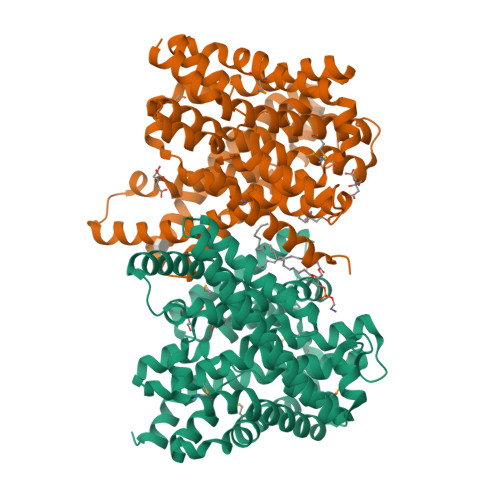
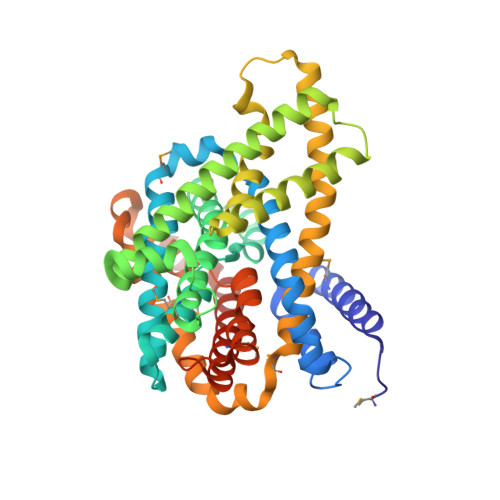
Structure and Substrate Ion Binding in the Sodium/Proton Antiporter Panhap.
Wohlert, D., Kuhlbrandt, W., Yildiz, O.(2014) Elife 3: 03579
- PubMed: 25426802
- DOI: https://doi.org/10.7554/eLife.03579
- Primary Citation of Related Structures:
4CZ8, 4CZ9, 4CZA - PubMed Abstract:
Sodium/proton antiporters maintain intracellular pH and sodium levels. Detailed structures of antiporters with bound substrate ions are essential for understanding how they work. We have resolved the substrate ion in the dimeric, electroneutral sodium/proton antiporter PaNhaP from Pyrococcus abyssi at 3.2 Å, and have determined its structure in two different conformations at pH 8 and pH 4. The ion is coordinated by three acidic sidechains, a water molecule, a serine and a main-chain carbonyl in the unwound stretch of trans-membrane helix 5 at the deepest point of a negatively charged cytoplasmic funnel. A second narrow polar channel may facilitate proton uptake from the cytoplasm. Transport activity of PaNhaP is cooperative at pH 6 but not at pH 5. Cooperativity is due to pH-dependent allosteric coupling of protomers through two histidines at the dimer interface. Combined with comprehensive transport studies, the structures of PaNhaP offer unique new insights into the transport mechanism of sodium/proton antiporters.
Organizational Affiliation:
Department of Structural Biology, Max Planck Institute of Biophysics, Frankfurt am Main, Germany.