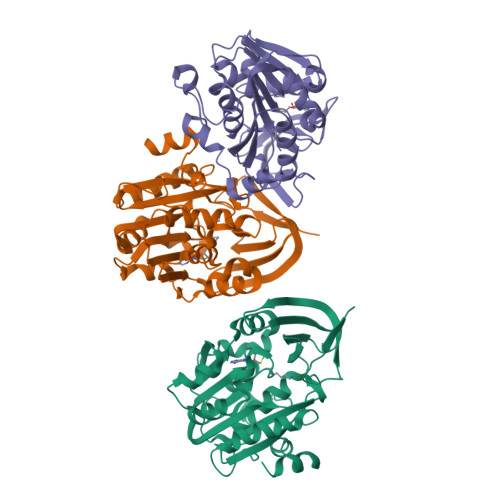
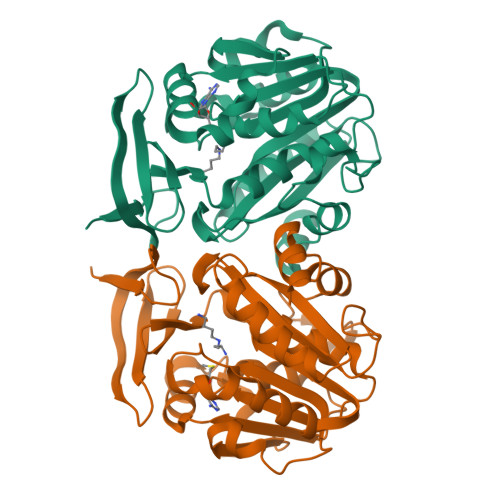
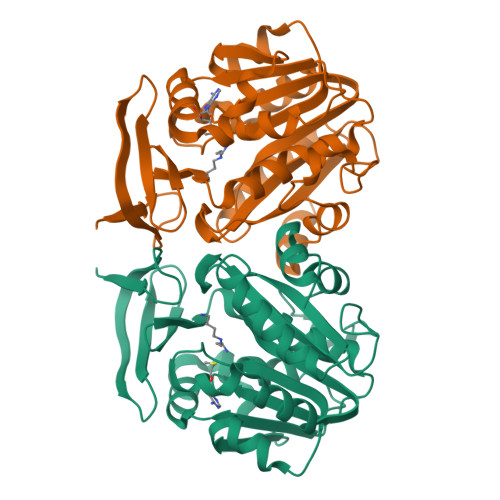
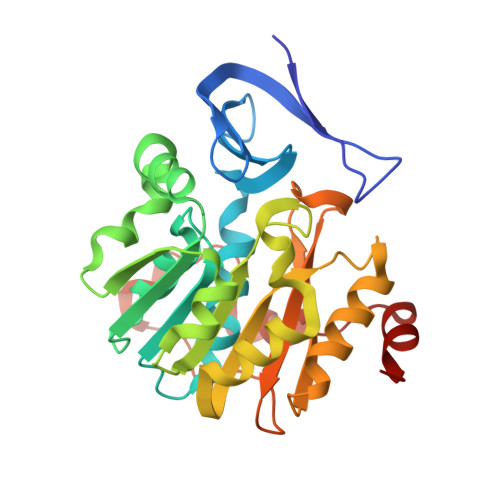
Three-Dimensional Structures of Plasmodium Falciparum Spermidine Synthase with Bound Inhibitors Suggest New Strategies for Drug Design.
Sprenger, J., Svensson, B., Halander, J., Carey, J., Persson, L., Al-Karadaghi, S.(2015) Acta Crystallogr D Biol Crystallogr 71: 484
- PubMed: 25760598
- DOI: https://doi.org/10.1107/S1399004714027011
- Primary Citation of Related Structures:
4BP1, 4BP3, 4CWA, 4CXM, 4UOE - PubMed Abstract:
The enzymes of the polyamine-biosynthesis pathway have been proposed to be promising drug targets in the treatment of malaria. Spermidine synthase (SpdS; putrescine aminopropyltransferase) catalyzes the transfer of the aminopropyl moiety from decarboxylated S-adenosylmethionine to putrescine, leading to the formation of spermidine and 5'-methylthioadenosine (MTA). In this work, X-ray crystallography was used to examine ligand complexes of SpdS from the malaria parasite Plasmodium falciparum (PfSpdS). Five crystal structures were determined of PfSpdS in complex with MTA and the substrate putrescine, with MTA and spermidine, which was obtained as a result of the enzymatic reaction taking place within the crystals, with dcAdoMet and the inhibitor 4-methylaniline, with MTA and 4-aminomethylaniline, and with a compound predicted in earlier in silico screening to bind to the active site of the enzyme, benzimidazol-(2-yl)pentan-1-amine (BIPA). In contrast to the other inhibitors tested, the complex with BIPA was obtained without any ligand bound to the dcAdoMet-binding site of the enzyme. The complexes with the aniline compounds and BIPA revealed a new mode of ligand binding to PfSpdS. The observed binding mode of the ligands, and the interplay between the two substrate-binding sites and the flexible gatekeeper loop, can be used in the design of new approaches in the search for new inhibitors of SpdS.
Organizational Affiliation:
Center for Molecular Protein Science, Lund University, SE-221 00 Lund, Sweden.