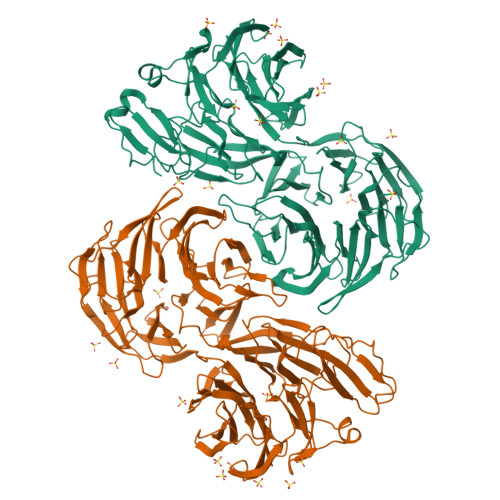
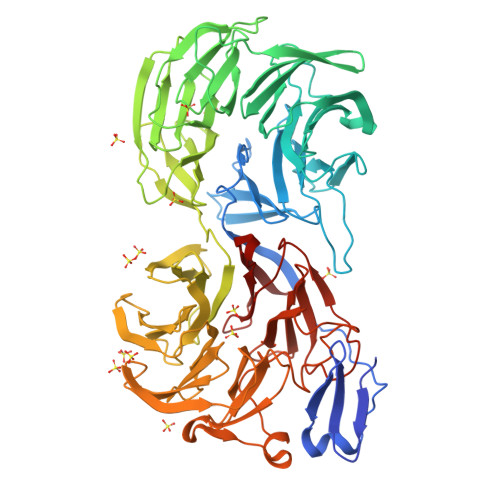
Crystal Structure of Eml1 Reveals the Basis for Hsp90 Dependence of Oncogenic Eml4-Alk by Disruption of an Atypical Beta-Propeller Domain.
Richards, M.W., Law, E.W.P., Rennalls, L.P., Busacca, S., O'Regan, L., Fry, A.M., Fennell, D.A., Bayliss, R.(2014) Proc Natl Acad Sci U S A 111: 5195
- PubMed: 24706829
- DOI: https://doi.org/10.1073/pnas.1322892111
- Primary Citation of Related Structures:
4CI8 - PubMed Abstract:
Proteins of the echinoderm microtubule-associated protein (EMAP)-like (EML) family contribute to formation of the mitotic spindle and interphase microtubule network. They contain a unique hydrophobic EML protein (HELP) motif and a variable number of WD40 repeats. Recurrent gene rearrangements in nonsmall cell lung cancer fuse EML4 to anaplastic lymphoma kinase (ALK), causing expression of several fusion oncoprotein variants. We have determined a 2.6-Å crystal structure of the representative ∼70-kDa core of EML1, revealing an intimately associated pair of β-propellers, which we term a TAPE (tandem atypical propeller in EMLs) domain. One propeller is highly atypical, having a discontinuous subdomain unrelated to a WD40 motif in place of one of its blades. This unexpected feature shows how a propeller structure can be assembled from subdomains with distinct folds. The HELP motif is not an independent domain but forms part of the hydrophobic core that joins the two β-propellers. The TAPE domain binds α/β-tubulin via its conserved, concave surface, including part of the atypical blade. Mapping the characteristic breakpoints of each EML4-ALK variant onto our structure indicates that the EML4 TAPE domain is truncated in many variants in a manner likely to make the fusion protein structurally unstable. We found that the heat shock protein 90 (Hsp90) inhibitor ganetespib induced degradation of these variants whereas others lacking a partial TAPE domain were resistant in both overexpression models and patient-derived cell lines. The Hsp90-sensitive EML4-ALK variants are exceptions to the rule that oncogenic fusion proteins involve breakpoints in disordered regions of both partners.
Organizational Affiliation:
Department of Biochemistry, University of Leicester, Leicester LE1 9HN, United Kingdom.